
- •Adhesion molecules
- •Naming names
- •Immunoglobulin superfamily
- •ICAM
- •SIGLEC
- •Junctional adhesion molecules (JAMs)
- •Claudins
- •Occludin
- •Integrins
- •Inactive to primed
- •Primed to active
- •Cadherins
- •Selectins
- •Cartilage link proteins
- •Integrins, cell survival, and cell proliferation
- •Inside-out signalling and the formation of integrin adhesion complexes
- •Outside-in signalling from integrin adhesion complexes
- •Integrins and cell survival
- •Integrins and cell proliferation
- •References

Signal Transduction to and from Adhesion Molecules
the membrane and the cytoskeleton,68 but it remains to be shown that they actually relay signals from CD44 into the cell. Another point of interest is of course, the capacity of CD44v3 to bind growth factors of the FGF and HGF/SF families. This may mean that it is involved in the recruitment of the respective receptors, thereby mediating intracellular signalling. In the following sections, signalling to and from CD44 will not be further discussed.
Integrins, cell survival, and cell proliferation
Inside-out signalling and the formation of integrin adhesion complexes
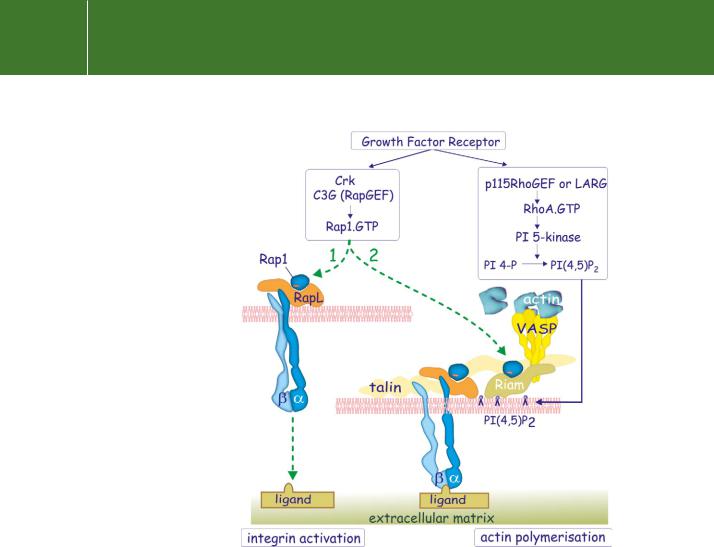
Cells organize their contacts with the extracellular matrix in the form of integrin clusters (see Figure 13.15a, b). These are composed of a number of proteins, some with structural and others with signalling roles. Formation of such focal adhesion sites involves activation of integrins, their binding to a component of the extracellular matrix (fibronectin, vitronectin, laminin, or collagen), the formation of actin filaments, and their attachment to these newly formed filaments. This process also requires the monomeric GTPases Rap1 and RhoA.
Rap1 (see page 251) contributes to integrin activation, and more generally it is a key regulator for the adhesion of blood borne cells and platelets.69,70 The mechanism of activation is shown in Figure 13.14. Rap1 is activated
by the guanine nucleotide exchange factor C3G71 recruited to tyrosinephosphorylated receptor tyrosine kinases (PDGFR or EGFR) via the SH2/SH3adaptor protein Crk (see pages 325, 326). Fibroblasts deficient in C3G display impaired adhesion and accelerated migration.72 Loading Rap1 with GTP allows an interaction with two effectors that play a role in integrin activation. One of these is RapL, which associates with the -chain of integrins.73 Mice lacking RapL show impaired transendothelial migration because their integrins fail to attach to the endothelial cells (see Chapter 16). The other effector is Riam which, in lymphocytes, localizes Rap1-GTP to adhesion structures,74 through its interaction with PI(4,5)P2. Riam then recruits VASP to the membrane, so setting the stage for actin polymerization.
RhoA mediates its effect in the assembly process through the activation of PI 5-kinase, generating PI(4,5)P2 (Figure 13.14 and Figure 13.15). As a result, both Riam and vinculin attach to the plasma membrane. In the case of vinculin, this unmasks cryptic binding sites that allow it to form a cross-bridge between talin (which is linked to the -subunit of integrins) and to -actinin (which
is linked to filamentous actin)75 (Figure 13.15c). A solid structure, integrating extracellular matrix and actin cytoskeleton, is thus formed.
397
Signal Transduction
FIG 13.14 Growth-factor-mediated activation of integrins. Tyrosine phosphorylated growth factor receptors bind the adaptor Crk that links to the guanine nucleotide exchange factor C3G and activates Rap1. This addresses two effectors, RapL and Riam. RapL binds the cytosolic domain of the -subunits of integrins and is implicated in their activation. Riam is recruited to the membrane by inositol lipids and binds VASP, leading to the initiation of actin polymerization. The growth factor also causes activation of the RhoA guanine exchange factors 115RhoGEF or LARG. These activate PI 5-kinase with production of PI(4,5)P2.
Outside-in signalling from integrin adhesion complexes
Anchorage dependent growth and survival
An essential requirement for the proliferation of tissue cells driven by growth factors is their attachment to a suitable surface. The fact that cells must adhere to substrate provides a likely molecular explanation for the anchorage-dependent growth of cells that form part of a solid tissue. Adherence-dependent signalling targets have much in common with
398
Signal Transduction to and from Adhesion Molecules
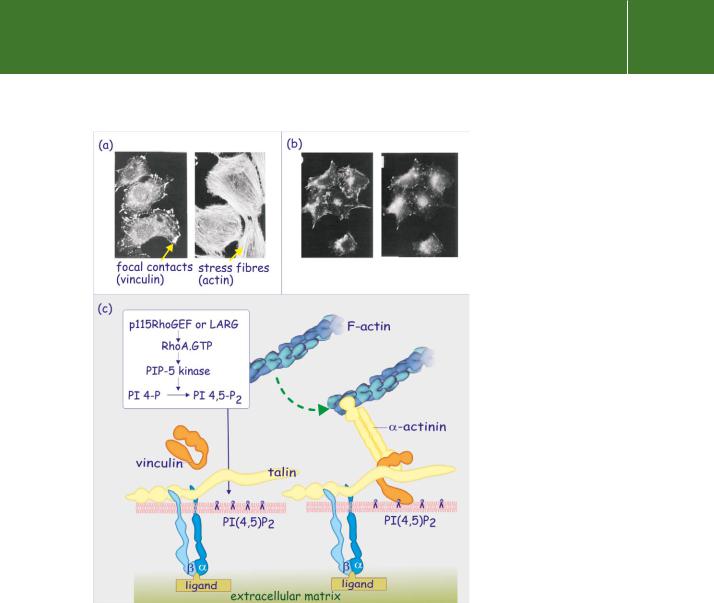
FIG 13.15 RhoA-mediated formation of focal contact sites. (a) The role of RhoA in focal contact formation is illustrated in micrographs of cells stained with fluorescent phalloidin (specifically binds to actin) or with antibodies against vinculin. In the presence of matrix proteins and serum, the fibroblasts spread out, forming
stress fibres and focal adhesion sites. (b) However, when a non-functional RhoA is introduced, actin stress fibres are absent and strong focal contacts are not formed. Adapted from Clark et al.76 (c) Growth factors play an essential role in the formation of focal adhesion sites through activation of the Rho nucleotide exchange factors p115RhoGEF or LARG. RhoA-GTP promotes formation of PI(4,5)P2 through activation of PI 5-kinase. Vinculin binds to the PI(4,5)P2, causing it to open up so that it attaches to both talin and -actinin, making the link between the integrins and the actin cytoskeleton.
399

Signal Transduction
One might expect that circulating cells, such as leukocytes, must have specialized mechanisms to protect them from apoptosis. For sure, some white blood cells such as neutrophils are very short-lived (just a few hours in the blood), but others, such as the lymphocytes responsible for immunological memory, survive for years. Whether memory cells need to be continually reminded
to survive by occasional encounters with other cells or an extracellular matrix remains to be established. Interestingly, lymphocytes employ
an alternative pathway to activate PKB which involves the GTPase Rac.79
growth factor signalling targets (described in Chapters 12 and 18) and they are seemingly redundant. Signalling targets are evident in various stages of the Ras-MAPkinase pathway; upstream of Ras, between Ras and Raf or between Raf and MEK. Other targets are the JNK pathway, STAT-mediated regulation of transcription and the PKB pathway. For all of these, adherence
and growth factor signalling act in synergy, the one amplifying the other, and this is necessary to engage the cell in a round of proliferation. The survival of endothelial and epithelial cells also depends critically upon the contacts they make with the extracellular matrix (and with each other). Without contact, they die through the controlled process of apoptosis, which in this particular context is referred to as ‘anoikis’ (meaning homeless).77,78
Cells have an intrinsic drive to self-destruct, but are prevented from doing so by signals emanating from specific rescue pathways. One such signal (outsidein) follows from the attachment of the integrins to the extracellular matrix. This mechanism may have evolved to protect the organism against stray cells colonizing inappropriate locations (metastasis, see page 307). The section below illustrates integrin-dependent signalling pathways that control cell survival and early events in the cell cycle (G1 phase).
Integrins and cell survival
The formation of an integrin signalling complex
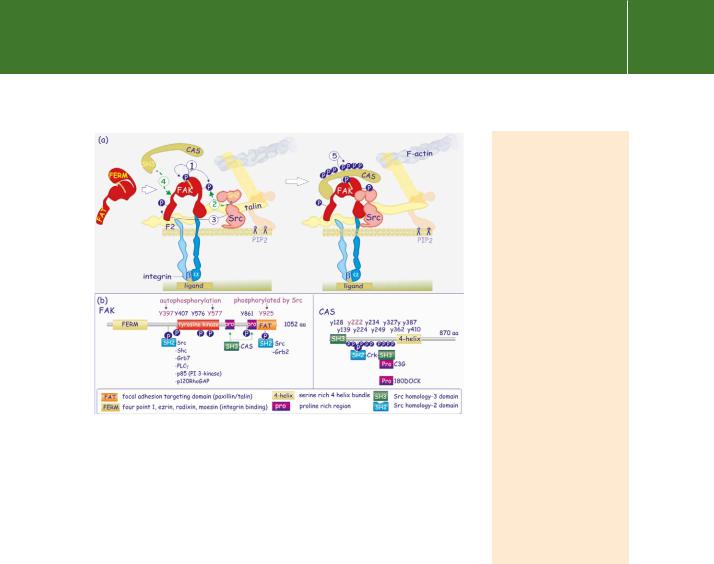
Focal adhesion complexes form the sites of attachment for the tyrosine kinase FAK (focal adhesion kinase) (see Figure 13.16). Although a focal adhesion targeting domain has been described,80 it is unclear which structural component acts as the docking site for FAK. Both paxillin and talin have been suggested. Attachment, resulting in autophosphorylation and activation, enables FAK to act as a docking site for Src (and also other members of the Src family of kinases such as Fyn and Yes). Src phosphorylates further tyrosine residues converting FAK into an SH2 domain docking protein. Besides binding SH2 domains, FAK also possesses two proline rich regions one of which interacts with the SH3 domains of CAS81 (see Figure 13.16). CAS, with its multiple tyrosine phosphorylation sites, acts as a docking protein attracting numerous SH2-containing effectors and adaptors (a role similar to IRS-1/2
for the insulin receptor or LAT in the case of TCR signalling). A lack of CAS prevents integrin-mediated FAK signalling.82
The importance of FAK is underlined by the finding that cells expressing a constitutively active form survive in suspension even though they are
effectively ‘homeless’.85 Here, FAK is active regardless of the failure to make contact with an extracellular matrix.
FAK-mediated activation of PKB
Amongst the proteins that attach to tyrosine phosphorylated FAK is the p85-regulatory subunit of PI 3-kinase (bound to the catalytic subunit p110)
400
Signal Transduction to and from Adhesion Molecules
FIG 13.16 A two-step process to create an integrin signalling complex. (a) With the structural components of the focal adhesion site assembled, the focal adhesion kinase FAK associates with the F2 lobe of the FERM
domain of talin; (Fak also has a FERM domain). Autophosphorylation FAK then generates a docking site (Y397) for the SH2 domain of Src which phosphorylates at Y925, in the FAT domain). Src and FAK next phosphorylate the FAK-associated docking protein CAS at multiple sites. An integrin-signalling complex is formed that acts in a manner similar to growth factor-receptor signalling complexes, i.e. attachment of adaptors and effectors and tyrosine phosphorylation of substrate. (b) Illustration of the main domains and essential phosphorylation sites of FAK and CAS.
(Figure 13.17). This generates 3-phosphorylated inositol lipids with the subsequent activation of the Ser/Thr kinase PKB (see Chapter 18).
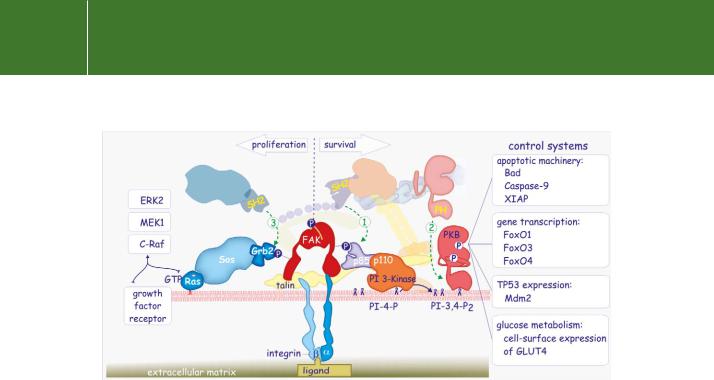
PKB effects a number of phosphorylations86–88 that promote rescue by at least four different mechanisms (see Figure 13.17). The first is by direct
phosphorylation and inactivation of components of the apoptotic machinery, including Bad, Caspase-9, and XIAP.89 Phosphorylation of Caspase-9 prevents its proteolytic activation while phosphorylation of Bad shifts its location from the mitochondrial membranes to the cytosol due to sequestration by 14-3-3 proteins. Since the phosphorylation site of human Caspase-9 is not conserved in mouse and rat it seems unlikely that this is a major control mechanism.
PKB also acts to control transcription of a number of genes that regulate apoptosis by phosphorylating the three members of the FoxO subfamily of Forkhead transcription factors (FoxO1, FoxO3, and FoxO4, previously known as FKHR, FKHRL1, and AFX) causing them to be retained in the cytosol,
The CAS protein family
– CAS (Crk-associated substrate) and EFS (embryonal Fynassociated substrate) – are SH3-containing docking proteins with multiple tyrosine phosphorylation sites that interact with SH2 domains of Crk (adaptor), Nck, and Abl (tyrosine protein kinases). In humans, CAS was identified as a protein whose over-expression confers resistance to anti-oestrogen treatment (tamoxifen) of breast cancer (BCAR1).83 CAS is an important mediator of Src-mediated cell transformation. CAS phosphorylation correlates with inhibition of expression of Fhl1, a transcription factor. Loss of Fhl1 is a marker of anchorage-independent cell growth.84
401
Signal Transduction
FIG 13.17 Survival and proliferation. The focal adhesion site promotes cell proliferation signals through activation of Ras. The Src phosphorylated Tyr925 acts as a binding site for the SH2 domain of Grb2. This interaction recruits the Ras guanine nucleotide exchange factor Sos to the membrane, leading to activation of Ras (1). Ras-GTP initiates the activation of the Raf-ERK pathway, necessary for initiation of the cell cycle. The focal adhesion site promotes cell survival signals through activation of the serine/threonine protein kinase-B (PKB). The phosphorylated Tyr residue of focal adhesion kinase (FAK) provides a binding site for the SH2 domain of the regulatory subunit (p85) of PI 3-kinase (2). Subsequent production of PI(3,4)P3 provides a binding site for the PH domain of PKB (3). After its activation by phosphorylation, PKB phosphorylates a large number of proteins that directly or indirectly deal with cell death (see text for further detail).
again by 14-3-3 proteins. In this way, they are prevented from activating genes critical for induction of factors that promote cell death such as the Fas ligand,90 TRAIL,91 TRADD, and the pro-apoptotic protein Bim.92 That this constitutes an essential survival mechanism is indicated by the finding that over-expression of FoxO3 triggers a programme of cell death in the
interleukin-3-dependent haematopoietic cell line Ba/F3.93 The death process is identical to that which occurs after withdrawal of the cytokine interleukin-3 (normally essential for these cells’ survival).
Another substrate of PKB, Mdm2, is a component of an E3 ubiquitin ligase complex (see page 469) that ubiquitylates the tumour suppressor protein p53, preparing it for destruction by the proteasome. Mdm2-assisted destruction of p53 occurs in cells that have undergone DNA damage (‘genotoxic stress’), but which seek to resist apoptosis. Phosphorylation of Mdm2 by PKB causes its nuclear localization and renders p53 ubiquitylation more efficient.94 As with caspase-9, this mechanism of protection by PKB is operational in human cells, although the phosphorylation sites are not conserved throughout mammalian species.
PKB also protects against apoptosis by intervening in carbohydrate metabolism. When cells are deprived of growth factors, nutrient uptake is
402
