
- •Adhesion molecules
- •Naming names
- •Immunoglobulin superfamily
- •ICAM
- •SIGLEC
- •Junctional adhesion molecules (JAMs)
- •Claudins
- •Occludin
- •Integrins
- •Inactive to primed
- •Primed to active
- •Cadherins
- •Selectins
- •Cartilage link proteins
- •Integrins, cell survival, and cell proliferation
- •Inside-out signalling and the formation of integrin adhesion complexes
- •Outside-in signalling from integrin adhesion complexes
- •Integrins and cell survival
- •Integrins and cell proliferation
- •References
Signal Transduction
Catenins were discovered in a search for proteins that associate with uvomorulin. Three proteins of 102, 88, and 80 kDa were repeatedly coimmunoprecipitated and found to be present in mouse, chicken and human cells. The were named catenin ( , , andrespectively), from the Latin catena, meaning chain, since one major function was thought to be linking the adhesion molecule with the cytoskeleton.47
Selectin: homologous to the Ca2 -dependent (C-type) lectin. Lectin is derived from the word ‘select’, and originally applied to plant proteins that bind to specific carbohydrate residues present on nitrogenfixing bacteria. They have been used in cell biological work because they bind to specific glycosidic residues present in the Golgi system and on cell surfaces. Only later was it found that animal cells also possess similar
proteins, generally on the surfaces of endothelial and myeloid cells.
the formation and maintenance of stable adhesions. Formin is involved in the polymerization of actin, which indicates that cadherin core complexes contribute to the elongation of the actin filaments to which they bind.45 The juxtamembrane region of cadherin also binds p120ctn (or -catenin), involved
in the recruitment of cytosolic protein tyrosine phosphatases of various sorts.46
In developmental processes, expression of each cadherin subclass is regulated both spatially and temporally and this is associated with individual morphogenic events. Thus, the tendency of cells to segregate or to aggregate correlates with the expression of particular cadherins. This is particularly apparent during gastrulation and the formation of the neural tube. In addition, regulation can also occur through activation, as occurs at the onset of compaction (see Figure 13.9) or in the process of axon guidance where local changes in cadherin adhesion steer the growth cone. Axon guidance cues, like Neurocan and Slit, rapidly inactivate cadherin through the phosphorylation of its intracellular partner -catenin.48,49 By eliminating cadherin-mediated traction, these axon guiding cues allow for growth cone extension. Many of the cadherins are implicated in long-term potentiation, memory formation, and spatial learning, most likely through their capacity to form synaptic contacts and thus spatially organize the brain cortical layer.50
In epithelial cells, cadherins are present in cell–cell junctions, adherens junctions, and desmosomes (Figure 13.11), being associated with bundles of actin filaments or intermediate filaments via the intermediates of -catenins and -catenin or plakoglobin and desmoplakin respectively. By forming cell– cell contacts, the cadherins appear to act as tumour suppressors and when these fail, as in metastasizing epithelial tumours, there is a loss of basolateral localization (see also Chapter 14).
Selectins
Selectins, present on the surface of white blood cells, platelets, and also the endothelial cells that line blood vessels, form a family of adhesion molecules (the selectin/LECAM family, leukocyte–endothelial cell adhesion molecules). They mediate the initial low-affinity adhesion sites for lymphocytes and
for leukocytes such as neutrophils. This prepares the way for cell migration into the lymph nodes and tissues. At the N-terminus there is a C-type (Ca2 - binding) lectin domain that enables binding to particular carbohydrate residues (galactose, N-acetyl glucosamine, and fucose) present on cell surface glycoproteins and glycolipids. They also contain a single EGF-like domain and a series of Sushi repeats (also referred to as complement regulatory proteins, CRP) (Figure 13.12).
Lymphocytes can be displaced from lymph nodes by monosaccharides (l-fucose and d-mannose) and by the polysaccharide fucoidin (rich in l-fucose). This gives an indication that carbohydrate residues may be involved in the
392

Signal Transduction to and from Adhesion Molecules
FIG 13.11 Epithelial adherent junctions. (a) Epithelial cells are firmly attached to each other by adherens junctions which play an important role in maintaining both cellular and tissue integrity. (b) Adherens junctions are formed by homophilic interactions between cadherins and they are connected to the cellular adhesion belt formed of actin filaments. Desmosomes are formed by homophilic interactions of desmogleins (or desmocollins), linked to intermediate filaments (keratin in the case of epithelial cells). At the basal membrane these filaments form a hemidesmosome, interacting with integrins via plectin. (c) The tight association between cadherin,-catenin, -catenin, and filamentous actin illustrates the physical stress-resisting quality of cell junctions.
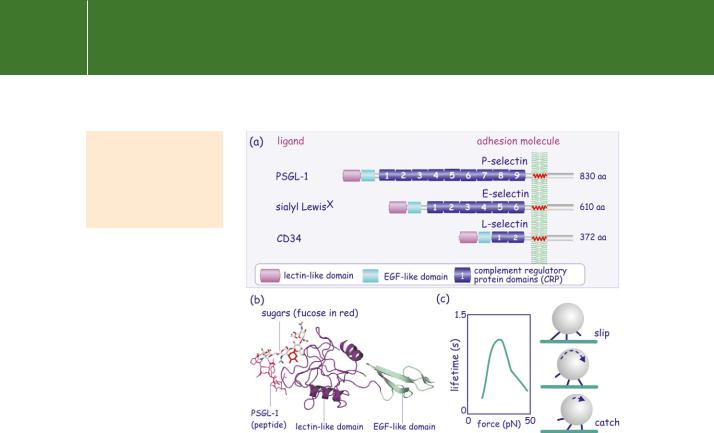
homing of lymphocytes.51 This was confirmed by the finding of a lymph- node-specific homing receptor which possesses a C-type lectin domain.52 Various selectins, mediating intercellular interactions, have since been identified in vascular endothelial cells (E), platelets (P), and leukocytes (L) (Figure 13.12a). l-Selectin is responsible for lymphocyte homing and is expressed on all circulating leukocytes except for a subpopulation of memory cells. It recognizes CD34 (a heavily glycosylated mucin) on endothelial cells. E- and P-selectins are expressed on endothelial cells and recognize, respectively, sialyl LewisX and PSGL-1 on leukocytes. P-selectin and PSGL-1 interact in a dimeric mode; two P-selectins bind to one PSGL-1.
393
Signal Transduction
sialyl Lewis: these are sialylated sugars
expressed on blood cells that serve as antigens in blood group typing as developed by Lewis.
FIG 13.12 Domain structure of selectins. (a) The selectins are characterized by the N-terminal lectin-homology domain and a variable number of complement regulatory protein domains (CRPs). Through their lectin domain, selectins interact with sugar residues present in cell surface glycoproteins and glycolipids. (b) Ribbon representation of the lectin-like domain of E-selectin bound to PSGL-1. Sugars in stick representation; fucose is
red. (c) The lifetime of the bonding of P-selectin to PSGL-1 increases with force, then decreases. This may explain the rolling of white blood cells (that express P-selectin) on the vascular endothelium (that expresses PSGL-1) under shear stress (blood flow). Image adapted from Yago et al.53 and Marshall et al.54 (1g1s55).
Selectins are recognized by their lectin-like domain (LD) which binds sugars (Figure 13.12b). This has the remarkable property that allows its affinity for ligands to be modulated by the imposition of shear forces.56 The interaction between L-selectin and PSGL-1 shows a biphasic relationship by which low shear forces decrease off-rates, the bond lifetime being prolonged (‘catch bonds’), while higher shear forces increase off-rates (‘slip bonds’). It appears, although the molecular details remain uncertain, that shearing deforms the interacting molecules such that they lock more tightly.54 This behaviour, catch and slip, might explain the rolling of leukocytes on the vascular endothelial layer under flow conditions (Figure 13.12).57
Little is known about the role of the selectins or the selectin-binding molecules in signal transduction. Several binding partners including-actinin, calmodulin, and members of the ezrin/radixin/moesin (ERM)
394

Signal Transduction to and from Adhesion Molecules
family of cytoskeletal proteins attach to the short cytoplasmic tail (17–35 residues) of selectins. With respect to extracellular ligands, PGSL-1 induces tyrosine phosphorylation by Syk, mediated by moesin. This pathway leads to transcriptional activation of the SRE element.58
Cartilage link proteins
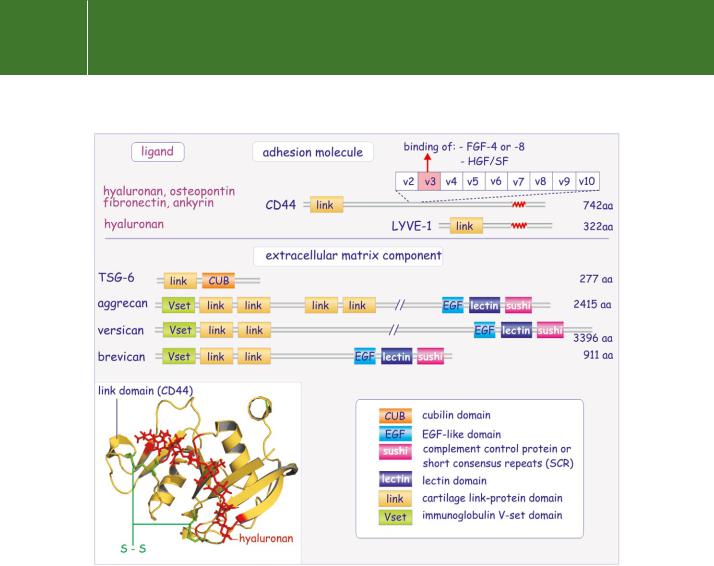
The glycosaminoglycan hyaluronan is a high molecular weight polysaccharide present in the tissue matrix and body fluids of all vertebrates. It plays a fundamental role in regulating cell migration and differentiation.59 The majority of hyaluronan-binding proteins belong to the cartilage link protein superfamily. All contain a conserved link module ( 100 residues) that has a three-dimensional structure resembling the sugar-binding domain of E-selectin (Figure 13.13). They can be further subdivided into two groups, those that are true cellular adhesion molecules such as CD4460,61 and LYVE-1,62
and those that are components of the extracellular matrix such as cartilage link protein itself, aggrecan, versican, brevican, and TSG-6.63 This second group plays an important role in the architecture of the extracellular matrix, bringing together the pressure-resistant qualities of glycosaminoglycans and the tension-resistant qualities of the extracellular matrix proteins (collagen, elastin), jointly, an essential quality of cartilage in articulated joints.
CD44, originally discovered as the homing receptor of lymphocytes, is the best studied of the cartilage link proteins. It is required when lymphocytes bind to high endothelial venules and exit the circulation, seeking lymph nodes.66 In a manner reminiscent of the integrins expressed on circulating blood cells, CD44 is functionally inactive. It binds hyaluronan only after T cell receptor triggering or exposure of the lymphocytes to inflammatory cytokines such as TNFand IFN- . In this way, selected lymphocytes are directed to inflammatory sites by binding to hyaluronan present on the surface of the vascular endothelial cells.
CD44 is a single membrane-spanning protein encoded by 19 exons of which nine in the extracellular domain (v2–v10) are variably spliced (Figure 13.13). Because of the extensive glycosylation and the sequence variability, the molecular mass of CD44 can vary widely, anything between 85 and 200 kDa. The link module is implicated in the binding of hyaluronan but the splicing extends the range of binding capacities. For instance, the inclusion of exon v3 allows the attachment of heparan or chondroitin sulfate, enabling it to interact with fibroblast growth factor-4 or -8 (FGF-4 or -8) and with hepatocyte growth factor/ scatter factor (HGF/SF). Other potential ligands are osteopontin, fibronectin, and ankyrin. Homophilic intercellular interactions with CD44 are also reported.
The splice variants stimulated great interest when it appeared that CD44v4–7 might determine metastasis in rat pancreatic tumour cells. In short, it seemed
Glycosaminoglycans and the extracellular matrix proteins, in particular collagen, share with wood and reinforced concrete the valuable qualities of resistance
to the forces of both tension and compression. These are two important requirements for the construction not only
of organisms but also of buildings.
395
Signal Transduction
FIG 13.13 Domain structure of the cartilage link proteins. Cartilage link proteins come in two forms, those that are transmembrane proteins and act as receptors, and those that are extracellular matrix proteins and play an important role in connecting glycosaminoglycans with proteins. They are characterized by the link domains that bind hyaluronan. CD44 has the capacity to form many splice variants (inserted variable exons v2–v10), which provide new binding properties independent of the link domain. The inset shows a ribbon representation of the link domain, stabilized by two disulfide bonds, connecting CD44 to a short stretch of hyaluronan (2bvk64 and 1poz65).
at first to have the quality of a metastasis gene product.67 More than this, a monoclonal antibody raised against v6 prevents metastasis. Unfortunately however, although human tumours often express splice variants of CD44 and in certain instances this predicates poor prognosis, there seem to be no functional implications. The signal transduction pathway emanating from CD44 remains uncertain. A number of membrane proteins, including ezrin, radixin, and moesin, that associate with it, belong to the group of erythrocyte band 4.1-related proteins. These are proposed to function as links between
396
