
- •Insulin receptor signalling; it took a little time to work out the details
- •Signalling through phosphoinositides
- •PI 3-kinase, PI(3,4)P2 and PI(3,4,5)P3
- •A family of PI 3-kinases
- •Studying the role of PI 3-kinase
- •Protein kinase B and activation through PI(3,4)P2
- •Insulin: the role of IRS, PI 3-kinase, and PKB in the regulation of glycogen synthesis
- •From the insulin receptor to PKB
- •From PKB to glycogen synthase
- •The role of PI 3-kinase in activation of protein synthesis
- •Other processes mediated by the 3-phosphorylated inositol phospholipids
- •So, who did discover insulin?
- •References

Signal Transduction
Unfortunately, it has all too readily been assumed that the actions of wortmannin are specific to the 3-kinases, even though it was in use as an inhibitor of myosin light chain kinase for at least
a couple of years before its first application as an inhibitor of PI 3-kinases.30 At concentrations above 10 7 M, wortmannin also inhibits a form of
PI 4-kinase and then, in the micromolar range, in addition to its effects on myosin light chain kinase, it possibly inhibits other protein kinases as well. As a result of its uncritical
use, the 3-phosphorylated lipids were considered to be implicated in several processes that are quite innocent of any such relationship.
This could be taken as an object lesson in the use of inhibitors in general.
Experience advises that the terms‘potent’ and‘highly specific’, frequently used to promote pharmacological agents on their first outing, may wear a bit thin after a year or two.
As the evidence of side effects accumulates, new ‘potent’ and‘highly specific’ agents come to take their place. The application of ‘potent’ and‘highly specific’ inhibitors of calmodulin and later of protein kinase C muddled progress in an earlier generation.
identified as a toxic agent causing acute necrosis of lymphoid tissues, severe myocardial haemorrhage and haemoglobinuria.27 At an appropriate dose, it is also a powerful anti-inflammatory agent.28
At concentrations above 10 9 M, wortmannin associates covalently with the p110 catalytic subunit of PI 3-kinase29 (Figure 18.3). Used judiciously, and in parallel with other inhibitors (e.g. Lilly compound LY294002) and independent approaches, wortmannin certainly has its place in the battery of techniques for investigating signal transduction. In the quest for inhibitory effects, however, it has often been applied at high concentrations, leading to false positive reports of inhibition. Furthermore, due to the existence of multiple isoforms of PI 3-kinase, not all of which are targets of this compound, a negative result does not necessarily rule out a role for the products of 3-phosphorylation.
Pathways of activation for PI 3-kinase
The process by which receptors for growth factors activate the type IA PI 3-kinases through interaction with the SH2 domain of the p85 regulatory subunits is well established (see Figure 18.4). PI 3-kinase can also be recruited and activated by non-receptor tyrosine kinases such as those of the Src family through the same mechanism.32 In platelets that have been stimulated by thrombin, it appears that activation of PI 3-kinase is linked to FAK. This kinase, associated with integrin signalling and cytoskeletal organization at focal adhesion sites, contains proline-rich regions that interact with the SH3 domain on p85.33 In addition, phosphorylation of FAK allows interaction with the SH2 domain of p85 and this appears to be the route of activation following attachment of cells to solid substrates. Apparently, there are two
routes to the activation of PI 3-kinase. One of these is direct, the other indirect through the involvement of focal adhesion sites (for further discussion concerning the link between FAK and the cytoskeleton (see page 400). PI 3- kinase is also activated by Ras which interacts with directly with the catalytic p110 subunit (Figure 18.4).21 Other GTPases, particularly those of the Rho family, are also involved as regulators (and downfield effectors) in pathways regulated by PI 3-kinase.23
Protein kinase B and activation through PI(3,4)P2
The viral oncogene v-akt (acutely transforming retrovirus AKT8) encodes a fusion product of a cellular serine/threonine kinase and the viral structural component Gag. This kinase, PKB, is similar to both PKC (73% identity to the catalytic domain) and PKA (68%). Unlike the other kinases, it contains a PH domain that enables it to bind to polyphosphoinositide head groups. There are three subtypes, all of which show a broad tissue distribution (Figure 18.5).
550

Phosphoinositide 3-Kinases, Protein Kinase B, and Signalling through Insulin Receptor
FIG 18.3 Inhibition of PI 3-kinase- by wortmannin and LY294002.
(a) The structure of the catalytic domain of PI 3-kinase resembles that of the classical protein kinases (Chapter 24). It is composed of a smaller N-lobe and a larger C-lobe and between them the catalytic cleft that harbours ATP and forms the substrate entry point. The activation segment (dotted orange line) determines the affinity of the kinase for phosphatidylinositol lipids. (b) Structure of PI 3-kinase showing the Ras-binding domain (RBD) (blue), C2 domain
(yellow), helical domain (green), and catalytic domain (pink). The RBD contacts the N-lobe and to a lesser degree the C-lobe, suggesting that Ras binding will have an allosteric (stimulatory) effect on the catalytic domain. The C2 domain participates in membrane interaction. The helical domain is common to the PI 3-kinase and PI 4-kinase families and provides the framework for the attachment of the other domains. (c) Wortmannin (W) binds covalently at K833 involved in coordination of the -phosphate of ATP. It irreversibly blocks access of ATP and thus kinase activity. (d) LY294002 (LY) also occupies the ATP binding pocket. Its predominant sites of interaction are at M804, Y812, and M953. Only the catalytic cleft of the kinase domain is shown and the structure lacks the N-terminal regions that interact with the regulatory subunit (p85 or p55) (1e8x,16 1e7u,16 1e7v31).
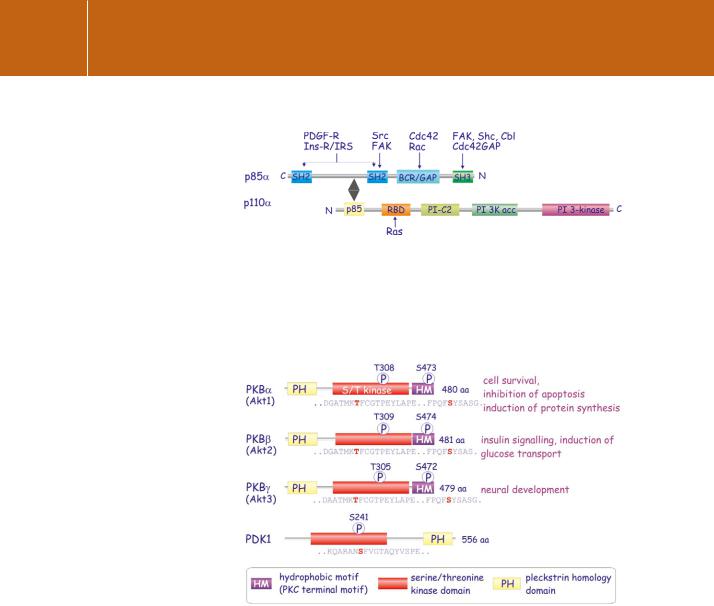
With respect to their structural organization and mode of activation, they belong to the AGC subfamily of serine/threonine protein kinases.
Shortly after its discovery, it was found that PKB is activated by PI(3,4)P2 but the actual mechanism of activation turns out to be far from simple. The
phospholipid plays two distinct roles, acting both as recruiting sergeant and then as an activating signal. The first is direct, the lipid head group binding to
The terms PKB and Akt both occur in the
literature: see text box on page 526. The PKB/Akt kinases are assembled in the SwissProt database under the ‘RAC’ subfamily of serine/threonine protein kinases because the mammalian sequence was discovered as a kinase related to PKA and PKC.34
The AGC family of protein kinases comprises serine/threonine kinases having structural homology with PKA, PKG, and PKC, hence the name AGC. This classification is not based on sequence homology. They contain a C-terminal hydrophobic motif (FxxF[S/T]Y) (except for PDK1). They also share a mode of activation in which (for most of them), PDK1 acts as the masterswitch. Examples are members of the S6Kinase subfamily (S6K1, RSK
and MSK1), members of protein kinase C family (PKC , 1, 2, ,, , and PRK), Serum-
glucocorticoid responsive kinase (SGK1) PDK1 and PKB , , 35 (for domain architecture of some of these protein kinases see Figures 18.7).
551
Signal Transduction
FIG 18.4 Multiple pathways to activate PI 3-kinase class 1 A.
Several proteins interact with the p85 regulatory subunit of PI 3-kinase. These include members of Rac and Cdc42 (Rho-related GTPases) that interact at the BCR/GAP domain. The receptors for PDGF and insulin, insulin receptor substrate (IRS), and also FAK and Src all interact at the SH2 domains. A proline-rich region present in FAK, Shc, Cbl, and Cdc42GAP interacts with the SH3 domain. The catalytic subunit p110 can interact directly with Ras. Domains denoted as in Figure 18.2.
FIG 18.5 Classification of the protein kinases B/Akt.
Originally identified as the oncogene in the transforming retrovirus AKT8, there are multiple isoforms of PKB/Akt ( , , and ). They all contain a PH domain and a hydrophobic motif at the C-terminus but differ slightly in the locations of their regulatory phosphorylation sites PKD1 is not a member of the PKB family but plays an important role in their activation.
the PH domain in the N-terminal segment of PKB.36 The other interaction is indirect, involving the soluble kinase PDK1, also endowed with a PH domain.37 Binding of PI(3,4)P2 is crucial since it enables PDK1 and PKB to embrace, an encounter that, as shown below, proves beneficial to both.
Two changes are required in order to render PKB catalytically competent. Firstly, the C-helix becomes structured through the interaction with the serine phosphorylated C-terminal hydrophobic motif (HM) (Figure 18.6a, b).
552

Phosphoinositide 3-Kinases, Protein Kinase B, and Signalling through Insulin Receptor
FIG 18.6 Activation of PKB .
(a) In the inactive PKB structure, the various regions of the kinase domains comprising the C-helix of the N-lobe and the activation segment are disordered. Substrate and ATP do not bind. The organization of the C-terminal segment with its hydrophobic motif (HM, shown in yellow) is indicated approximately. (1gzk40). (b) Binding of the C-terminal hydrophobic motif (HM, yellow) with the C-helix is facilitated by phosphorylation of S474. This induces reorganization of the C-helix (pink) and a second phosphorylation in the activation segment (T309) organizes the activation segment (red). Binding of ATP and substrate ensues (2jdr41). (c). Binding of the hydrophobic motif to the C-helix (1) leads to structural rearrangements in which E200 engages and correctly positions K180 (2) that coordinates the binding of ATP in the catalytic cleft. The H196 of the ordered C-helix also engages the (PDK) phosphorylated T309 resulting in a reorientation of the activation segment (3). The kinase now binds both ATP and substrate and is fully competent to phosphorylate substrate.(2jdr41).
Then, again following phosphorylation, the activation segment is reorganized (Figure 18.6b,c). Two separate kinases are required. Unlike most kinases, PDK1 is constitutively (but only partially) active. Also, unlike the other kinases of this class, having no intrinsic hydrophobic (HM) motif, the activating impetus is provided by its substrates, which, in return, become phosphorylated
(Figure 18.7). The full activation signal requires a priming phosphorylation in the C-terminal domain catalysed by the tentatively named PDK2. Its identity remains elusive. The kinase complex mTOR/Rictor (mTORC2) is among the favourite candidates. Unless both buttons are pressed on PKB, little happens.38 The need for 3-phosphoinositides to activate PKB may go beyond that of colocalization with PDK1 since binding of its PH domain to PI(3,4)P2 induces
a conformational change that facilitates phosphorylation of the activation segment by PDK1.39
553
