
Gale Encyclopedia of Genetic Disorder / Gale Encyclopedia of Genetic Disorders, Two Volume Set - Volume 1 - A-L - I
.pdf
Prognosis
The prognosis for an individual with Down syndrome is quite variable, depending on the types of complications (heart defects, susceptibility to infections, development of leukemia, etc.). The severity of the retardation can also vary significantly. Without the presence of heart defects, about 90% of children with Down syndrome live into their teens. People with Down syndrome appear to go through the normal physical changes of aging more rapidly, however. The average age of death for an individual with Down syndrome is about 50 to 55 years.
Still, the prognosis for a baby born with Down syndrome is better than ever before. Because of modern medical treatments, including antibiotics to treat infections, and surgery to treat heart defects and duodenal atresia, life expectancy has greatly increased. Community and family support allows people with Down syndrome to have rich, meaningful relationships. Because of educational programs, some people with Down syndrome are able to hold jobs.
As of early 2001, there has only been one report of a male affected with Down syndrome becoming a father. Approximately 60% of women with Down syndrome are fully capable of having children. The risk of a woman with trisomy 21 having a child affected with Down syndrome is 50%.
Resources
BOOKS
Pueschel, Siegfried M. A Parent’s Guide to Down Syndrome:
Toward a Brighter Future. Revised ed. New York: Paul H. Brookes Publishing Co., 2000.
Selikowitz, Mark. Down Syndrome: The Facts. 2nd ed. London: Oxford University Press, 1997.
Stray-Gunderson, K. Babies with Down Syndrome: A New Parents’ Guide. Kensington: Woodbine House, 1986.
PERIODICALS
Carlson, Tucker, and Jason Cowley. “When a Life is Worth Living: Down’s Syndrome Children.” The Times (29 November 1996): 18 .
Cohen, William, ed. “Health Care Guidelines for Individuals with Down Syndrome: 1999 Revision.” Down Syndrome Quarterly (September 1999).
Hattori, M., A. Fujiyama, D. Taylor, H. Watanabe, et al. “The DNA sequence of human chromosome 21.” Nature (18 May 2000): 311–19.
ORGANIZATIONS
National Down Syndrome Congress. 7000 PeachtreeDunwoody Rd., Bldg 5, Suite 100, Atlanta, GA 303281662. (770) 604-9500 or (800) 232-6372. Fax: (770) 604-9898. ndsccenter@aol.com. http://www.ndsccenter
.org .
National Down Syndrome Society. 666 Broadway, New York, NY 10012-2317. (212) 460-9330 or (800) 221-4602. Fax: (212) 979-2873. http://www.ndss.org info@ndss.org .
WEBSITES
Down Syndrome Health Issues. http://www.ds-health.com/ . (15 February 2001).
Down Syndrome Information Network. http://www.downsyndrome.net/ . (15 February 2001).
Down Syndrome WWW Page.http://www.nas.com/downsyn/ . (15 February 2001).
Recommended Down Syndrome Sites on the Internet.http://www.ds-health.com/ds_sites.htm#natl . (15 February 2001).
Paul A. Johnson
DRPLA see Dentatorubral-pallidoluysian atrophy
I Duane retraction syndrome
Definition
Duane retraction syndrome is a congenital disorder that limits the movement of the eye. It may also involve other systems of the body.
Description
Duane retraction syndrome (DRS or DURS) is an inherited disorder characterized by a limited ability to move the eye to one side or the other. DRS is congenital, meaning that it is present at birth. It results from abnormal connections among the nerves that control the muscles of the eyes. About 80% of DRS cases involve one eye (unilateral) and about 20% involve both eyes (bilateral). Most unilateral DRS cases (72%) involve the left eye.
DRS was first described in 1905 by A. Duane. It also is known as:
•Duane syndrome (DUS)
•DR syndrome
•eye retraction syndrome
•retraction syndrome
•Stilling-Turk-Duane syndrome
DRS is one of a group of conditions known as strabismus, or misalignment of the eye. DRS is classified as an incomitant strabismus, because it is a misalignment of
syndrome retraction Duane
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
353 |
Duane retraction syndrome
the eye that varies depending on the direction that the eye is gazing. It is further classified as an extraocular muscle fibrosis syndrome. This means that it is a condition associated with the muscles that move the eyes. Both the active and the passive movement of the eyeball are affected in DRS.
Physiology
DRS is believed to result from an abnormality that occurs during the development of the fetus in the womb. It may be caused by either environmental or genetic factors, or a combination of both. The developmental abnormality is believed to occur between the third and eighth weeks of fetal development. This is the period when the ocular muscles that rotate the eye, and the cranial nerves from the brain that control the ocular muscles, are forming in the fetus.
DRS appears to result from the absence of cranial nerve VI, which is known as the abducens nerve. The nerve cells in the brain that connect to the abducens nerve are also missing. The abducens nerve controls the lateral rectus muscle of the eye. This muscle moves one eye outward toward the ear, as a person looks toward that side. This movement is called abduction. In DRS, the nerves from a branch of cranial nerve III (the oculomotor nerve) also are abnormal. The oculomotor nerve controls several eye muscles, including the medial rectus muscle. This muscle moves the eye inward toward the nose, as the person looks toward the other side. This movement is called adduction.
The majority of individuals with DRS have limited or no ability to move an eye outward toward the ear. Instead, the opening between the eyelids of that eye widens and the eyeball protrudes. In addition, individuals with DRS may have only a limited ability to move the eye inward, toward the nose. Instead, when looking inward toward the nose, the medial and lateral recti muscles contract simultaneously. This causes the eyeball to retract, or pull into the skull, and causes the opening between the eyelids to narrow, as if one were squinting. Sometimes, the eye moves up or down as the individual attempts to look in toward the nose. This is called upshoot or downshoot, respectively.
In some individuals with DRS, the eyes may cross when looking straight ahead. Gazing straight ahead is called the primary position or primary gaze. Crossed eyes may cause the person to turn the head to one side or the other, to restore binocular vision. In such individuals, this “head turn” may become habitual.
Associated syndromes
About 30-50% of individuals with DRS have associated abnormalities. These may include additional eye
problems, deafness, and nervous system or skeletal abnormalities. In particular, DRS may be associated with abnormalities in the upper extremities, especially the hands. Sometimes DRS is associated with Holt-Oram syndrome, a hereditary heart defect.
Okihiro syndrome is DRS in association with other abnormalities that may include:
•flatness in the normally-fleshy region between the thumb and the wrist (the thenar eminence) of one or both hands
•inability to flex the joint in the thumb
•hearing loss or deafness in one or both ears
Okihiro syndrome also is known as:
•Duane syndrome with radial ray anomalies (as in the arms and hands)
•Duane/radial dysplasia syndrome (referring to abnormal tissue growth in the arms and hands)
•DR syndrome (the “D” refers to Duane anomaly and deafness; the “R” refers to radial and renal (kidney) dysplasia, or abnormal tissue growth in the arms, hands, and kidneys)
•Duane anomaly with radial ray abnormalities and deafness
Genetic profile
The genetic basis of DRS is unclear. The specific gene or genes that are responsible for DRS and the associated syndromes have not been identified. DRS may arise from a combination of environmental factors and defects in one or more genes.
Portions of several of the 23 pairs of human chromosomes may be associated with DRS. A gene that is involved in DRS has been localized to a region of chromosome 2. Deletions of portions of chromosomes 4 and 8 have also been associated with DRS. The presence of an additional small chromosome, thought to be broken off from chromosome 22, has been associated with DRS. It is possible that these chromosome rearrangements and abnormalities may account for the wide range of symptoms and syndromes that can occur with DRS.
The inheritance of DRS is autosomal, meaning that the trait is not carried on either the X or Y sex chromosomes. The most common type of DRS, DRS1, is inherited as an autosomal dominant trait. This means that only a single copy of a DRS gene, inherited from one parent, can result in the condition. The offspring of a parent with DRS is expected to have a 50% chance of inheriting the disorder. However, the autosomal dominant form of DRS sometimes skips a generation in the affected family; for example, a grandparent and grandchildren may have
354 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

DRS, but the middle generation does not. Some forms of DRS may be recessive, requiring two copies of a gene, one inherited from each parent.
Family members may exhibit different types of DRS, indicating that the same genetic defect may be expressed by a range of symptoms. The severity of DRS also may vary among family members. Furthermore, the majority of individuals with DRS do not appear to have a family history of the disorder. There are very few reports of single families with a large number of affected individuals. However, close relatives of individuals with DRS often are affected by some of the other abnormalities that may be associated with the disorder.
Okihiro syndrome, or Duane syndrome with radial ray anomalies, and Holt-Oram syndrome both are inherited as autosomal dominant traits. However, like DRS, Okihiro syndrome may skip a generation in a family, or may be expressed by a range of symptoms within one family.
Demographics
DRS is estimated to affect 0.1% of the general population. It accounts for 1-5% of all eye movement disorders. Although it is not a sex-linked disorder, females are more likely than males to be affected by DRS (60% compared with 40%).
Signs and symptoms
Types of DRS
There are three generally-recognized types of DRS. Type 1 DRS (DRS1) accounts for about 70% of cases. With DRS1, abduction, the ability to move the eye toward the ear, is limited or absent. The eye widens and the eyeball protrudes when the eye is moved outward. In contrast, adduction, the ability to move the eye toward the nose, is normal or almost normal. However, the eye narrows and the eyeball retracts during adduction. The eyes of infants and children with DRS1 are usually straight ahead in the primary position. However, some children develop an increasing misalignment in the primary position and may compensate by turning their head.
With DRS type 2, adduction is limited or absent but abduction is normal, or only slightly limited. The eye narrows and the eyeball retracts during adduction. Type 2 accounts for approximately 7% of DRS cases.
With DRS Type 3, both abduction and adduction are limited. The eye narrows and the eyeball retracts during adduction. Type 3 accounts for about 15% of DRS cases.
Each type of DRS is subclassified, depending on the symptoms that occur when the individual is looking
K E Y T E R M S
Abducens nerve—Cranial nerve VI; the nerve that extends from the midbrain to the lateral rectus muscle of the eye and controls movement of the eye toward the ear (abduction).
Abduction—Turning away from the body.
Adduction—Movement toward the body. In Duane retraction syndrome, turning the eye inward toward the nose.
Autosomal dominant—A pattern of genetic inheritance where only one abnormal gene is needed to display the trait or disease.
Congenital—Refers to a disorder which is present at birth.
Downshoot—Downward movement of the eye.
Dysplasia—The abnormal growth or development of a tissue or organ.
Extraocular muscle fibrosis—Abnormalities in the muscles that control eye movement.
Head turn—Habitual head position that has been adopted to compensate for abnormal eye movements.
Holt-Oram syndrome—Inherited disorder characterized by congenital heart defects and abnormalities of the arms and hands; may be associated with Duane retraction syndrome.
Lateral rectus muscle—The muscle that turns the eye outward toward the ear (abduction).
Medial rectus muscle—The muscle that turns the eye inward toward the nose (adduction).
Oculomotor nerve—Cranial nerve III; the nerve that extends from the midbrain to several of the muscles that control eye movement.
Okihiro syndrome—Inherited disorder characterized by abnormalities of the hands and arms and hearing loss; may be associated with Duane retraction syndrome.
Primary position, primary gaze—When both eyes are looking straight ahead.
Recessive—Genetic trait expressed only when present on both members of a pair of chromosomes, one inherited from each parent.
Strabismus—An improper muscle balance of the ocular musles resulting in crossed or divergent eyes.
Upshoot—Upward movement of the eye.
syndrome retraction Duane
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
355 |
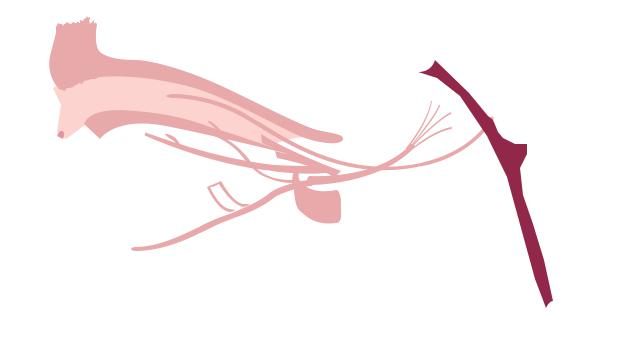
syndrome |
|
Duane retraction |
III |
IV |
|
VI |
|
|
CN VI |
Absence of cranial nerve VI (dashed line) is indicative of Duane retraction syndrome and results in abnormal head and eye movements. (Gale Group)
straight ahead (primary gaze). With subgroup A, the eye turns in toward the nose when gazing ahead. With subgroup B, the eye turns out toward the ear during a primary gaze. With subgroup C, the eyes are straight ahead in the primary position.
Associated symptoms
The majority of individuals with DRS are healthy and have no other symptoms. However, other body systems that may be affected with DRS include:
•skeleton
•ears and hearing
•additional involvement of the eyes
•nervous system
With Okihiro syndrome, the DRS can be unilateral or bilateral. In addition to a flatness at the base of the thumb, there may be difficulty with thumb movements. There also may be abnormalities or the complete absence of the radial and ulnar bones of the forearm. In extreme cases, the thumb or forearm may be absent. Okihiro syndrome may be accompanied by hearing loss, abnormal facial appearance, and heart, kidney, and spinal abnormalities.
Sometimes Wildervanck syndrome is associated with DRS. This syndrome may include congenital deafness and a fusion of the cervical (neck) vertebrae (C2 and C3).
Diagnosis
Diagnosis of DRS usually occurs by the age of ten. The clinical evaluation includes a complete family history, an eye examination, and examinations for other eye involvement or other physical abnormalities.
Eye examinations include the following measurements:
•visual acuity or sharpness
•alignment of the eyes
•range of motion of the eyes
•retraction (pulling in) of the eyeballs
•size of the eye opening between the eyelids
•upshoots and downshoots
•head turns
Hearing tests are frequently conducted. The cervical (neck) and thoracic (chest) parts of the spine, the vertebrae, the hands, and the roof of the mouth all are included in the examination as well.
Treatment and management
Special glasses with prisms can eliminate the head turning that is associated with DRS. Vision therapy may help with secondary vision problems.
356 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

Surgery may be performed for the following cosmetic reasons:
•abnormalities in the primary gaze (when looking straight ahead)
•an unusual compensatory head position
•a large upshoot or downshoot
•severe retraction of the eye
The goal of surgery is to reduce or eliminate the misalignment of the eye that causes abnormal head turning, as well as to reduce the retraction of the eyeball and the upshoots and downshoots. The surgery is directed at the affected muscles of the eye.
Children with DRS, as well as their siblings, require complete medical examinations to detect other abnormalities that may be associated with DRS.
Prognosis
If children with DRS go undiagnosed, a permanent loss of vision may occur. Surgical procedures may eliminate head turns and improve the misalignment of the eyes, particularly in the primary position. However, the absence of nerves for controlling the muscles of the eye cannot be corrected. Thus, no surgical procedure can completely eliminate the abnormal eye movements. However, the condition does not get worse during the course of one’s life.
Resources
BOOKS
Engle, E. “The Genetics of Strabismus: Duane, Moebius, and Fibrosis Syndromes.” In Genetic Diseases of the Eye: A
Textbook and Atlas. Edited by E. Traboulsi, 477–512. New York: Oxford University Press, 1998.
PERIODICALS
Appukuttan, B., et al. “Localization of a Gene for Duane Retraction Syndrome to Chromosome 2q31.” American
Journal of Human Genetics 65 (1999): 1639–46.
Chung, M., J.T. Stout, and M.S. Borchert. “Clinical Diversity of Hereditary Duane’s Retraction Syndrome.” Ophthalmology 107 (2000): 500–03.
Evans, J.C., T.M. Frayling, S. Ellard, and N.J. Gutowski. “Confirmation of Linkage of Duane’s Syndrome and Refinement of the Disease Locus to an 8.8-cM Interval on Chromosome 2q31.” Human Genetics 106 (2000): 636–38.
ORGANIZATIONS
American Association for Pediatric Ophthalmology and Strabismus. http://med-aapos.bu.edu/ .
Genetic Alliance. 4301 Connecticut Ave. NW, #404, Washington, DC 20008-2304. (800) 336-GENE (Help-
line) or (202) 966-5557. Fax: (888) 394-3937. info @geneticalliance. http://www.geneticalliance.org .
March of Dimes Birth Defects Foundation. 1275 Mamaroneck Ave., White Plains, NY 10605. (888) 663-4637 or (914) 428-7100. resourcecenter@modimes.org. http://www
.modimes.org .
National Eye Institute. National Institutes of Health. 31 Center Dr., Bldg. 31, Rm 6A32, MSC 2510, Bethesda, MD 20892-2510. (301) 496-5248. 2020@nei.nih.gov.http://www.nei.nih.gov/ .
Schepens Eye Research Institute. 20 Staniford St., Boston, MA 02114-2500. (617) 912-0100. http://www.eri.harvard
.edu .
WEBSITES
Cooper, Jeffrey. “Duane’s Syndrome.” All About Strabismus. Optometrists Network. 2001. (22 Apr. 2001).http://www.strabismus.org/Duane_Syndrome.html .
Duane’s Retraction Syndrome. Yahoo! Groups. 2001. (22 Apr. 2001). http://groups.yahoo.com/group/duanes .
The Engle Laboratory. Research: Duane Syndrome. Children’s Hospital Boston. (22 Apr. 2001). http://www.tch.harvard
.edu/research/engle/duane.html .
Margaret Alic, PhD
I Dubowitz syndrome
Definition
Dubowitz syndrome is a genetic disorder defined by slow growth, a characteristic facial appearance, and a small head.
Description
Dubowitz syndrome was first described in 1965 by the English physician Dr. Victor Dubowitz. This genetic disorder causes growth retardation both before and after birth. It is primarily diagnosed through the distinctive facial features of affected individuals, including a small triangular-shaped face with a high forehead and wide-set, slitted eyes. A number of other symptoms, most commonly irritation and itching of the skin (eczema), may be present in infants born with Dubowitz syndrome.
Genetic profile
Dubowitz syndrome is passed on through an autosomal recessive pattern of inheritance. Autosomal means that the syndrome is not carried on a sex chromosome, while recessive means that both parents must carry the
syndrome Dubowitz
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
357 |

Dubowitz syndrome
K E Y T E R M S
Eczema—Inflammation of the skin with redness and other variable signs such as crusts, watery discharge, itching.
Microcephaly—An abnormally small head.
Ptosis—Drooping of the upper eyelid.
gene mutation in order for their child to have the disorder. Parents with one child affected by Dubowitz syndrome have a 25% chance that their next child will also be affected with the disease.
As of 2001, the specific gene mutation responsible for Dubowitz syndrome had not yet been identified.
Demographics
Cases of Dubowitz syndrome have been reported from many different regions of the world with the majority coming from the United States, Germany, and Russia. There does not appear to be any clear-cut ethnic pattern to the incidence of the syndrome. Dubowitz syndrome appears to affect males and females with equal probability. The overall incidence of the disorder has not been established since it is very rare. As of 1996, only 141 cases had been reported worldwide.
Signs and symptoms
Physical characteristics
The symptoms of people diagnosed with Dubowitz syndrome vary considerably. However, the most common physical characteristics associated with Dubowitz syndrome are growth retardation, characteristic facial appearance, and a very small head (microcephaly). A wide variety of secondary physical characteristics may be present.
GROWTH RETARDATION Children born with Dubowitz syndrome usually have a low birth weight. Slower than normal growth continues after birth. Even if the infant is born in the normal range, the height and weight gradually falls toward the low end of growth curves during childhood. However, Dubowitz syndrome is not a form of dwarfism, because affected individuals have normally proportioned bodies.
FACIAL APPEARANCE The characteristic facial appearance of people with Dubowitz syndrome is the primary way in which the disorder is recognized. The face
is small and often triangular in shape with a pointed, receding chin. The nose is broad with a wide or rounded tip. The eyes are set far apart and sometimes appear slitted due to a decreased distance between top and bottom eyelids or a drooping top eyelid. The forehead is high, broad, and sloping. Eyebrows and hair are thin or absent. The ears may be abnormally shaped or placed.
MICROCEPHALY Infants born with Dubowitz syndrome have primary microcephaly, or a small head size at birth. By definition, in microcephaly the circumference of the head is in the second percentile or less, meaning that 98% or more of all infants have a larger head circumference than an infant with microcephaly.
OTHER PHYSICAL CHARACTERISTICS There are many other physical characteristics that have been observed in the majority of cases of Dubowitz syndrome, although they are not present in all affected individuals. These include:
•A soft or high-pitched cry or voice
•Partial webbing of the toes
•Cleft palate or less severe palate malformations
•Genital abnormalities, including undescended testicles
•Gastroesophophageal reflux
•Inflammation and itching of the skin (eczema)
Mental and behavioral characteristics
Despite the small head size of children born with Dubowitz syndrome, developmental delay is not observed in all cases. Estimates of the incidence of developmental delay in cases of Dubowitz syndrome range from 30% to 70%, and in most cases the level of the mental retardation is rather mild.
A number of behavioral characteristics have been described by parents of children with Dubowitz syndrome as well as in the medical literature. These include:
•Extreme hyperactivity
•Temper tantrums, difficulty in self-calming
•Preference for concrete thinking rather than abstract thinking
•Language difficulties
•Shyness and aversion to crowds
•Fondness for music and rhythm
Diagnosis
Since the genetic cause is not known, there is no specific medical test that can definitively assign the diagno-
358 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |

sis of Dubowitz syndrome. The diagnosis is usually based on the characteristic facial appearance of the affected individual as well as on other factors such as growth data and medical history. The diagnosis is easily missed if the physician is not familiar with genetic pediatric conditions.
Treatment and management
A number of chronic medical conditions are associated with Dubowitz syndrome. These include:
•Inflammation and itching of the skin (eczema)
•Susceptibility to viral infections
•Allergies
•Chronic diarrhea or constipation
•Feeding difficulties and vomiting
These conditions need to be managed individually with appropriate treatments. For example, skin creams containing corticosteroid drugs are used to treat eczema.
Other physical problems caused by Dubowitz syndrome, such as drooping eyelids (ptosis) or cardiovascular defects, can be corrected through surgery.
Prognosis
The prognosis for individuals affected by Dubowitz syndrome is good provided that management of their medical conditions is maintained. Dubowitz syndrome has not been reported to cause shortened lifespan or any degenerative conditions. People with Dubowitz syndrome can expect to survive to adulthood and lead a fairly normal lifestyle, although most have some level of mental retardation.
Resources
PERIODICALS
Tsukahara, M., and J. Opitz. “Dubowitz Syndrome: Review of 141 Cases Including 36 Previously Unreported Patients.”
American Journal of Human Genetics (1996): 277-289.
ORGANIZATIONS
Dubowitz Syndrome Nationwide Support Group Network. RR 1 Box 114, Downs, IL 61736. (309) 724-8407.
Dubowitz Syndrome Parent Support. PO Box 173, Wheatland, IN 47597. (812) 886-0575.
WEBSITES
Dubowitz Syndrome Information and Parent Support.
http://www.dubowitz.org/ (20 April 2001).
“Dubowitz Syndrome.” Online Mendelian Inheritance in Man.
http://www.ncbi.nlm.nih.gov/htbin-post/Omim/
dispmim?223370 (20 April 2001).
Paul A. Johnson
I Duchenne muscular dystrophy
Definition
The group of conditions called muscular dystrophies are characterized by muscle weakness and degeneration. Duchenne is a relatively common, severe muscular dystrophy. Becker muscular dystrophy is less common and less severe. Becker and Duchenne muscular dystrophy were once considered to be separate conditions. In the 1990s, researchers showed that Duchenne and Becker muscular dystrophy have the same etiology (underlying cause). However, the two disorders remain distinct based on different ages on onset, rates of progression, and some distinct symptoms.
Description
Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD) are both defined by progressive muscle weakness and atrophy. Both conditions are caused by a mutation in the same gene and usually affect only boys. Symptoms of Duchenne muscular dystrophy usually begin in childhood, and boys with DMD are often in wheelchairs by the age of 12 years. Symptoms of Becker muscular dystrophy begin later, and men with BMD typically do not require wheelchairs until their 20s.
Boys with Duchenne muscular dystrophy are usually diagnosed at a young age. Boys with Becker muscular dystrophy are often diagnosed much later. Both conditions are progressive, although DMD progresses more quickly than BMD. Unfortunately, no treatments exist to slow or prevent progression of the disease. Skeletal muscles are affected initially. Eventually the muscles of the heart are also affected, and both conditions are fatal. The life expectancy of males with Duchenne and Becker is 18 years and approximately 45 years, respectively. Both conditions are caused by disorders of the muscle, not of the nerves that control the muscle.
Genetic profile
Duchenne and Becker muscular dystrophy are both caused by mutations in the DMD gene on the X chromosome. This is an exceptionally large gene, and control of its expression is complex.
Humans each have 46 chromosomes, of which 23 are inherited from the mother and 23 are inherited from the father. The sets of 23 chromosomes are complimentary: each contains the same set of genes. Therefore, every human has a pair of every gene. Genes are the sequences of DNA that encode instructions for growth,
dystrophy muscular Duchenne
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
359 |
Duchenne muscular dystrophy
development, and functioning. One of the 23 pairs of chromosomes may not be complimentary: the sex chromosomes. Boys have an X chromosome and a Y chromosome. Girls have two X chromosomes.
Scientists often say that every person has the same genes, and that the genes on a pair of complimentary chromosomes are the same. It is true that a specific gene at a specific place on each chromosome provides the body with a very specific instruction, i.e. plays a particular functional role. However, most genes have multiple forms. Scientists call the various forms of a gene alleles. A given gene may have multiple alleles that function normally and multiple alleles that lead to physical problems.
Mutations (changes) in the DMD gene cause Duchenne and Becker muscular dystrophy. The DMD gene provides instructions for a protein called dystrophin. Mutations in DMD associated with Duchenne often completely disrupt production of dystrophin, such that no dystrophin is present. Mutations in DMD associated with Becker lead to a reduced amount of dystrophin being made and/or abnormal dystrophin. Certain mutations (alleles) in the DMD gene lead to the symptoms of DMD and other mutations lead to the symptoms of BMD.
Sex linked inheritance
Because the DMD gene is on the X chromosome, Duchenne and Becker muscular dystrophy affect only boys. Most females have two X chromosomes. Thus, if a female inherits an X chromosome with a mutation in the DMD gene, she has another normal DMD gene on her other X chromosome that protects her from developing symptoms. Women who have one mutated gene and one normal gene are called carriers. Boys, on the other hand, have an X and a Y chromosome. The Y chromosome has a different set of genes than the X chromosome; it mostly contains genes that provide instructions for male development. If a boy has a mutation in the DMD gene on his X chromosome, he has no normal DMD gene and he has muscular dystrophy.
If a woman has one son with Duchenne or Becker and no other family history, she may or may not be a carrier. If a woman has another family member with Duchenne or Becker muscular dystrophy, and a son with muscular dystrophy, it is assumed that she is a carrier. The risk for a male child to inherit the mutated gene from his carrier mother is 50% with each pregnancy. Based on the family history, geneticists can determine the likelihood that a woman is or is not a carrier. Based on this estimate, risks to have a son with muscular dystrophy can be provided.
New mutations
The DMD gene is very large and new mutations are fairly common. A new mutation is a mutation that occurs for the first time, that no other members have. Approximately 1/3 of males with Duchenne who have no family history of muscular dystrophy have the condition because of a new mutation that is only present in themselves. In this case, the affected male’s mother is not a carrier. Approximately 2/3 of males with Duchenne and no family history have it because of a new mutation that occurred in a relative. In other words, even if the affected male is the first in his family his mother may still be carrier. The new mutation could have happened for the first time in the affected male’s mother, or the new mutation could have occurred in his maternal grandmother or grandfather (or their parents, or their parents, etc.).
Sometimes a woman or man has mutations in the DMD gene of his or her sperm or eggs, but not in the other cells of his or her body. The mutation may even be in some sperm and/or eggs but not in others. This situation is called “germline mosaicism”. Germline cells are the egg and sperm cells. A woman or man with germline mosaicism may have more than one affected son even though genetic studies of his or her blood show that he or she is not a carrier. Geneticists can estimate the risk that a person has germline mosaicism, and provide information regarding the risk for a person with germline mosaicism to have a child with muscular dystrophy.
Demographics
Duchenne muscular dystrophy affects approximately 1/3,500 males. Males from every ethnicity are affected. Becker muscular dystrophy is much less common than Duchenne muscular dystrophy. The incidence of Becker muscular dystrophy is approximately 1/18,000.
Signs and symptoms
Both Becker and Duchenne muscular dystrophy initially affect skeletal muscle. Muscle weakness is the first symptom. Both conditions are progressive. Duchenne progresses more rapidly than Becker. People with Duchenne usually begin to use a wheelchair in their early teens, while people with Becker muscular dystrophy may not use a wheelchair until their twenties or later. In the late stages of both diseases, the cardiac muscles begin to be affected. Impairment of the heart and cardiac muscles leads to death. Some female carriers have mild muscle weakness.
People with muscular dystrophy often develop contractures. A contracture makes a joint difficult to move. The joint becomes frozen in place, sometimes in a
360 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

painful position. Scoliosis (curvature of the spine) is another common problem. Most people with Duchenne have normal intelligence, but cognition is affected in some. Cognition is not usually affected in Becker muscular dystrophy.
Dystrophin
The DMD gene contains instructions for a protein called dystrophin. Dystrophin is part of muscle cells and some nerve cells. Its function is not entirely understood. Based on its location in the muscle cell, scientists think that dystrophin may help maintain the structural integrity of muscle cells as they contract. People with Duchenne make very little or no dystrophin, and people with Becker make less than normal and/or semi-functional dystophin. When there is not enough dystrophin in the muscle, it becomes weak and starts to waste away. The muscle tissue is replaced by a fatty, fibrous tissue.
Duchenne muscular dystrophy
The first symptoms of Duchenne muscular dystrophy are usually noticed in early childhood. Delays in developmental milestones, such as sitting and standing, are common. The affected child’s gait is often a characteristic waddle or toe-walk. He often stumbles, and running is difficult. While parents notice these symptoms retrospectively, and may notice them at the time, muscular dystrophy often is not suspected until additional signs are apparent. By the age of four to five years, it is difficult for the child to climb stairs or rise from a sitting position on the floor. It is around this time that the diagnosis is usually made. A particular method, called the Gower sign is used by the child to raise himself from sitting on the floor. These motor problems are caused by weakness in large muscles close to the center of the body (proximal).
Although some muscles, such as the calves, appear to be large and defined, the muscle is actually atrophied and weak. It appears large because deposits of fatty, fibrous tissue are replacing muscle tissue. Enlarged calves are a characteristic sign of Duchenne muscular dystrophy, and are said have pseudohypertrophy. “Pseudo” means false, “hyper” is excessive, and “ trophy” is growth or nourishment. Other muscles may also have pseudohypertophy. These muscles feel firm if massaged.
The weakness begins at the center of the body (the pelvis) and progresses outward from the hips and shoulders to the large muscles of the legs, lower trunk, and arms. The weakness is symmetrical; i.e. both sides of the body are equally weak. Early signs of weakness, such as stumbling and difficulty climbing, progress to the point that the affected boy is unable to walk. Boys with
K E Y T E R M S
Cardiac muscle—The muscle of the heart.
Chromosome—A microscopic thread-like structure found within each cell of the body and consists of a complex of proteins and DNA. Humans have 46 chromosomes arranged into 23 pairs. Changes in either the total number of chromosomes or their shape and size (structure) may lead to physical or mental abnormalities.
Contracture—A tightening of muscles that prevents normal movement of the associated limb or other body part.
Mutation—A permanent change in the genetic material that may alter a trait or characteristic of an individual, or manifest as disease, and can be transmitted to offspring.
Scoliosis—An abnormal, side-to-side curvature of the spine.
Skeletal muscle—Muscles under voluntary control that attach to bone and control movement.
Translocation—The transfer of one part of a chromosome to another chromosome during cell division. A balanced translocation occurs when pieces from two different chromosomes exchange places without loss or gain of any chromosome material. An unbalanced translocation involves the unequal loss or gain of genetic information between two chromosomes.
X inactivation—Sometimes called “dosage compensation”. A normal process in which one X chromosome in every cell of every female is permanently inactivated.
Duchenne muscular dystrophy usually require wheelchairs by the age of 12 years. Eventually the muscles that support the neck are affected. The muscles of the digestive tract are affected in some males in the later stages of the disease. Contractures and scoliosis develop. Some boys also have learning disabilities or mild mental retardation.
Cardiac symptoms and life expectancy
The weakness usually affects skeletal muscles first, then cardiac muscle. Skeletal muscles are those that attach to bones and produce movement. The muscle weakness of both Duchenne and Becker muscular dystrophy progresses to affect the cardiac muscles. Weak, abnormal cardiac muscles cause breathing difficulties
dystrophy muscular Duchenne
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
361 |
Duchenne muscular dystrophy
and heart problems. Breathing difficulties lead to lung infections, such as pneumonia. These problems are fatal in Duchenne, and often fatal in Becker. The life expectancy for a boy with Duchenne muscular dystrophy is the late teens or early twenties. The average life expectancy of males with Becker muscular dystrophy is the mid-forties.
Becker muscular dystrophy
The initial signs of Becker muscular dystrophy may be subtle. The age at which symptoms become apparent is later and more variable than that of DMD. The progression of Becker muscular dystrophy is slower than that of DMD. Like Duchenne muscular dystrophy, boys with BMD develop symmetrical weakness of proximal muscles. The calf muscles often appear especially large. Boys with Duchenne muscular dystrophy develop weakness in the muscles that support their necks, but boys with BMD do not. The incidence and severity of learning disabilities and mild mental retardation is less in Becker muscular dystrophy than in Duchenne.
The first symptoms of Becker muscular dystrophy usually appear in the twenties and may appear even later. Weakness of the quadriceps (thigh muscle) or cramping with exercise may be the first symptom. The age of onset and rate of progression are influenced by how much dystrophin is made and how well it functions. Not all males with Becker muscular dystrophy become confined to wheelchairs. If they are, the age at which they begin to use the wheelchair is later than in Duchenne. Many males with Becker muscular dystrophy are ambulatory in their twenties. However, many males with Becker eventually develop cardiac problems, even if they do not have a great deal of skeletal muscle weakness. Cardiac problems are typically fatal by the mid-40s. Some men with Becker muscular dystrophy remain ambulatory (and alive) into their sixties.
Since Duchenne and Becker muscular dystrophy are caused by a mutation (change) in the same gene, the two conditions are usually distinguished based on age of onset and rate of progression. Males with Duchenne usually require wheelchairs by the age of 12 years and males with Becker usually do not require wheelchairs until after the age of 16. However, some males with muscular dystrophy develop symptoms at an intermediate age. Similarly, some males have elevated creatine kinase and abnormal muscle biopsies but do not develop most of the symptoms typical of muscular dystrophy. Some doctors would classify these males with very mild symptoms as having “mild Becker muscular dystrophy”. Some individuals who have Becker muscular dystrophy with mildly affected skeletal muscles still develop abnormalities of their cardiac muscle.
Many other forms of muscular dystrophy exist and are part of the diagnoses considered when a person develops signs of Duchenne or Becker muscular dystrophy. The symptoms of Becker muscular dystrophy, in particular, may be caused by many other conditions. However, diagnostic studies can definitively confirm whether an individual has Becker muscular dystrophy.
Affected females
It is unusual, but some females have some or all of the symptoms of muscular dystrophy. Assuming that the diagnosis is correct, this can happen for various reasons. If a woman has Turner syndrome, in which she has one X chromosome instead of two, she could also have Duchenne or Becker muscular dystrophy. (She has no second X chromosome with a normal DMD gene to protect her.) Alternatively, a woman may have muscular dystrophy because of random unfavorable “X inactivation”, or because she has a chromosomal translocation. Rarely, she may also have inherited both X chromosomes from the same parent.
Diagnosis
The diagnosis of muscular dystrophy is based on physical symptoms, family history, muscle biopsy, measurement of creatine kinase, and genetic testing. Creatine kinase (CK) may also be called creatine phosphokinase or CPK. It is a protein present in skeletal muscle, cardiac muscle, and the brain.
Creatine kinase is released into the blood as muscle cells die. The level of CK in the blood is increased if a person has muscular dystrophy. The level in a male with Duchenne is often more than ten times the normal level, and the level in a male with Becker is often at least five times more than the normal level. The level of CK in the blood of female carriers is variable. Approximately 50% of Duchenne muscular dystrophy carriers have slightly to greatly elevated serum creatine kinase. Only about 30% of carriers of Becker muscular dystrophy have elevated creatine kinase. Therefore, the measurement of creatine kinase is not an accurate predictor of carrier status.
If a muscle biopsy is performed, a small piece of muscle tissue is removed from the patient. Special studies are performed on the tissue. Early in the course of the disease, the muscle shows general abnormalities. Later in the disease, the muscle tissue appears more abnormal. The fat and fibrous tissues that are replacing the muscle fibers are visable.
Another specialized test of muscle function, the electromyogram (EMG) may be performed. The EMG records the electrical activity of a muscle. This test is used to determine whether the symptoms are the result of
362 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
