A Dictionary of Science
.pdf





881 |
|
|
|
|
|
Appendix 1 |
|
|
|
|
|
|
|
Appendix 1. SI units |
|
|
|
|
|
|
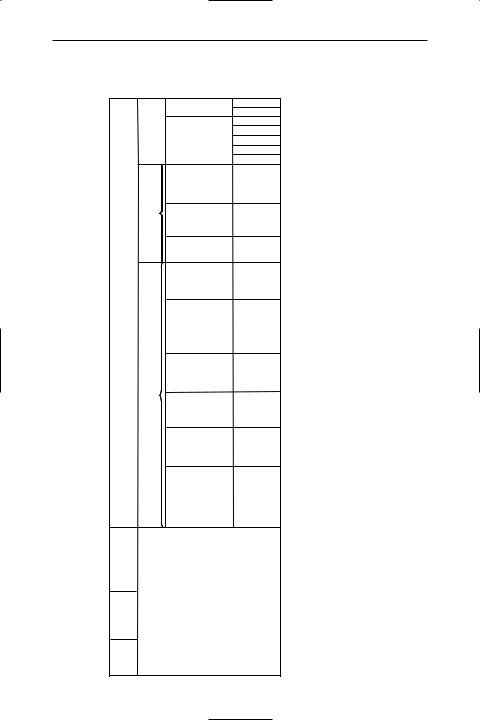
TABLE 1.1 Base and dimensionless SI units |
||||||
|
|
|
|
|
|
|
Physical quantity |
Name |
Symbol |
||||
|
|
|
|
|
|
|
length |
metre |
m |
||||
mass |
kilogram |
kg |
||||
time |
second |
s |
||||
electric current |
ampere |
A |
||||
thermodynamic temperature |
kelvin |
K |
||||
luminous intensity |
candela |
cd |
||||
amount of substance |
mole |
mol |
||||
*plane angle |
radian |
rad |
||||
*solid angle |
steradian |
sr |
||||
|
|
|
|
|
|
|
*dimensionless units |
|
|
|
|
|
|
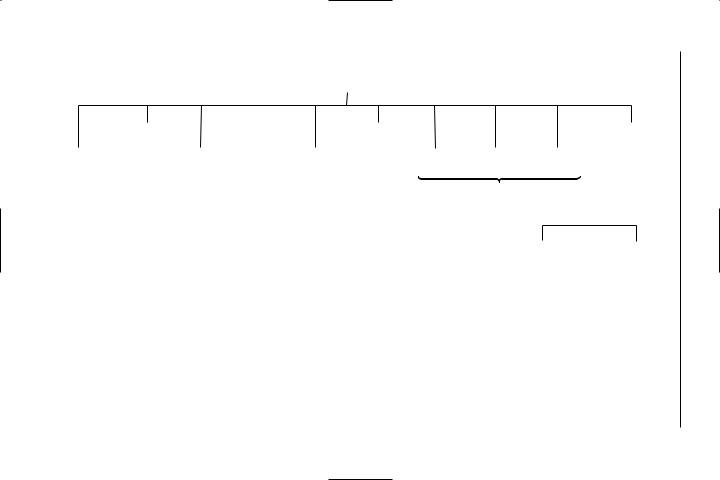
TABLE 1.2 Derived SI units with special names |
||||||
|
|
|
|
|
||
Physical quantity |
Name of |
Symbol of |
||||
|
SI unit |
SI unitAAI |
||||
|
|
|
|
|||
frequency |
hertz |
Hz |
||||
energy |
joule |
J |
||||
force |
newton |
N |
||||
power |
watt |
W |
||||
pressure |
pascal |
Pa |
||||
electric charge |
coulomb |
C |
||||
electric potential difference |
volt |
V |
||||
electric resistance |
ohm |
Ω |
||||
electric conductance |
siemens |
S |
||||
electric capacitance |
farad |
F |
||||
magnetic flux |
weber |
Wb |
||||
inductance |
henry |
H |
||||
magnetic flux density |
tesla |
T |
||||
(magnetic induction) |
|
|
|
|
|
|
luminous flux |
lumen |
lm |
||||
illuminance |
lux |
lx |
||||
absorbed dose |
gray |
Gy |
||||
activity |
becquerel |
Bq |
||||
dose equivalent |
sievert |
Sv |
||||
|
|
|
|
|
|
|

Appendix 1 |
882 |
TABLE 1.3 Decimal multiples and submultiples to be used with SI |
|||||||
units |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Submultiple |
Prefix |
Symbol |
Multiple |
Prefix |
Symbol |
||
|
|
|
|
|
|
|
|
10–1 |
deci |
d |
10 |
deca |
da |
||
10–2 |
centi |
c |
102 |
hecto |
h |
||
10–3 |
milli |
m |
103 |
kilo |
k |
||
10–6 |
micro |
µ |
106 |
mega |
M |
||
10–9 |
nano |
n |
109 |
giga |
G |
||
10–12 |
pico |
p |
1012 |
tera |
T |
||
10–15 |
femto |
f |
1015 |
peta |
P |
||
10–18 |
atto |
a |
1018 |
exa |
E |
||
10–21 |
zepto |
z |
1021 |
zetta |
Z |
||
10–24 |
yocto |
y |
1024 |
yotta |
Y |
|
|
TABLE 1.4 |
Conversion of units to SI units |
|||||
|
|
|
|
|
||
From |
To |
Multiply byAAJ |
||||
|
|
|
|
|
||
in |
m |
2.54 × 10–2 |
||||
ft |
m |
0.3048 |
|
|
|
|
sq. in |
m2 |
6.4516 × 10–4 |
||||
sq. ft |
m2 |
9.2903 × 10–2 |
||||
cu. in |
m3 |
1.63871 × 10–5 |
||||
cu. ft |
m3 |
2.83168 × 10–2 |
||||
l(itre) |
m3 |
10–3 |
|
|
|
|
gal(lon) |
l(itre) |
4.546 |
09 |
|
|
|
miles/hr |
m s–1 |
0.477 |
04 |
|
|
|
km/hr |
m s–1 |
0.277 |
78 |
|
|
|
lb |
kg |
0.453 |
592 |
|
|
|
g cm–3 |
kg m–3 |
103 |
|
|
|
|
lb/in3 |
kg m–3 |
2.767 |
99 |
× 104 |
|
|
dyne |
N |
10–5 |
|
|
|
|
poundal |
N |
0.138 |
255 |
|
|
|
lbf |
N |
4.448 |
22 |
|
|
|
mmHg |
Pa |
133.322 |
|
|
|
|
atmosphere |
Pa |
1.013 |
25 |
× 105 |
|
|
hp |
W |
745.7 |
|
|
|
|
erg |
J |
10–7 |
|
|
|
|
eV |
J |
1.602 |
10 |
× 10–19 |
||
kW h |
J |
3.6 × 106 |
|
|
|
|
cal |
J |
4.1868 |
|
|
|
|
|
|
|
|
|
|
|

883 |
|
|
|
Appendix 3 |
|
|
|||||
Appendix 2. Fundamental constants |
|||||
|
|
|
|
|
|
Constant |
Symbol |
Value in SI unitsAAAAAAAJJ |
|||
|
|
|
|
|
|
acceleration of free fall |
g |
9.806 |
65 m s–2 |
||
Avogadro constant |
L, NA |
6.022 |
1367(36) × 1023 mol–1 |
||
Boltzmann constant |
k = R/NA |
1.380 |
658(12) × 10–23 J K–1 |
||
electric constant |
ε0 |
8.854 |
187 817 × 10–12 F m–1 |
||
electronic charge |
e |
1.602 |
177 33(49) × 10–19 C |
||
electronic rest mass |
me |
9.109 |
3897(54) × 10–31 kg |
||
Faraday constant |
F |
9.648 |
5309(29) × 104 C mol–1 |
||
gas constant |
R |
8.314 |
510(70) J K–1 mol–1 |
||
gravitational constant |
G |
6.672 |
59(85) × 10–11 m3 kg–1 s–2 |
||
Loschmidt’s constant |
NL |
2.686 |
763(23) × 1025 m–3 |
||
magnetic constant |
µ0 |
4π × 10–7 H m–1 |
|||
neutron rest mass |
mn |
1.674 |
9286(10) × 10–27 kg |
||
Planck constant |
h |
6.626 |
0755(40) × 10–34 J s |
||
proton rest mass |
mp |
1.672 |
6231(10) × 10–27 kg |
||
speed of light |
c |
2.997 |
924 58 × 108 m s–1 |
||
Stefan–Boltzmann constant |
σ |
5.670 |
51(19) × 10–8 W m–2 K–4 |
||
|
|
|
|
|
|
Appendix 3. The solar system
Planet |
Equatorial |
Mean distance from |
SiderealAIAL |
||
|
diameter (km) |
sun (106 km) |
periodAKKIK |
||
|
|
|
|
|
|
Mercury |
4879.4 |
57.91 |
86.70 |
days |
|
Venus |
12 103.6 |
108.21 |
221.46 |
days |
|
Earth |
12 756.3 |
149.6 |
0.999 |
years |
|
Mars |
6794 |
227.94 |
677.0 |
days |
|
Jupiter |
142 985 |
778.41 |
11.86 |
years |
|
Saturn |
120 536 |
1426.72 |
29.42 |
years |
|
Uranus |
51 118 |
2870.97 |
83.75 |
years |
|
Neptune |
49 528 |
4498.25 |
163.72 |
years |
|
Pluto |
2390 |
5906.38 |
248.02 |
years |
|
|
|
|
|
|
|