
A Dictionary of Science
.pdf
viscose process |
856 |
forms cellulose Übres as rayon (artiÜcial silk).
viscose process See rayon.
viscosity A measure of the resistance to Ûow that a Ûuid offers when it is subjected to shear stress. For a *Newtonian Ûuid, the force, F, needed to maintain a velocity gradient, dv/dx, between adjacent planes of a Ûuid of area A is given by: F = ηA(dv/dx), where η is a constant, the coefÜcient of viscosity. In *SI units it has the unit pascal second (in the c.g.s. system it is measured in *poise). Non-Newtonian Ûuids, such as clays, do not conform to this simple model. See also kinematic viscosity.
visible spectrum The *spectrum of electromagnetic radiations to which the human eye is sensitive. See colour.
vision The sense that enables perception of objects in the environment by means of the *eyes.
visual acuity Sharpness of vision: the ability of the eye to distinguish between objects that lie close together. This hinges on the ability of the eye to focus incoming light to form a sharp image on the retina. Visual acuity depends on the *cone cells, which are most densely packed in the *fovea, close to the centre of the retina, and are therefore in the optimum position to receive focused light. In addition, each cone cell synapses with a single nerve cell and is thus able to send a separate signal, via the optic nerve Übres, to the brain.
visual binary See binary stars.
visual-display unit (VDU) The part of a
*computer system or word processor on which text or diagrams are displayed. It consists of a *cathode-ray tube and usu-
vally has its own input keyboard attached. visual purple See rhodopsin.
vital capacity The total amount of air that can be exhaled after maximum inspiration. The vital capacity of an average human is about 4.5 litres; in trained male athletes it can be 6 litres or more. However, some air always remains in the lungs (see residual volume).
vital staining A technique in which a
harmless dye is used to stain living tissue for microscopical observation. The stain may be injected into a living animal and the stained tissue removed and examined (intravital staining) or the living tissue may be removed directly and subsequently stained (supravital staining). Microscopic organisms, such as protozoa, may be completely immersed in the dye solution. Vital stains include trypan blue, vital red, and Janus green, the latter being especially suitable for observing mitochondria.
vitamin One of a number of organic compounds required by living organisms in relatively small amounts to maintain normal health. There are some 14 generally recognized major vitamins: the watersoluble *vitamin B complex (containing 9) and *vitamin C and the fat-soluble *vitamin A, *vitamin D, *vitamin E, and *vitamin K. Most B vitamins and vitamin C occur in plants, animals, and microorganisms; they function typically as *coenzymes. Vitamins A, D, E, and K occur only in animals, especially vertebrates, and perform a variety of metabolic roles. Animals are unable to manufacture many vitamins themselves and must have adequate amounts in the diet. Foods may contain vitamin precursors (called provitamins) that are chemically changed
to the actual vitamin on entering the body. Many vitamins are destroyed by light and heat, e.g. during cooking. See Chronology.
vitamin A (retinol) A fat-soluble vitamin that cannot be synthesized by mammals and other vertebrates and must be provided in the diet. Green plants contain precursors of the vitamin, notably carotenes, that are converted to vitamin A in the intestinal wall and liver. The aldehyde derivative of vitamin A, retinal, is a constituent of the visual pigment *rhodopsin. DeÜciency affects the eyes, causing night blindness, xerophthalmia, and eventually total blindness. The role of vitamin A in other aspects of metabolism is less clear; it may be involved in controlling *ATP production and the growth of epithelial cells.
vitamin B complex A group of watersoluble vitamins that characteristically
857
VITAMINS
1897 |
Dutch physician Christiaan Eijkman (1858–1930) cures beriberi in |
|
chickens with diet of whole rice. |
1906–07 |
British biochemist Sir Frederick Hopkins demonstrates existence of |
|
accessory dietary elements essential for growth. |
1912 |
Polish-born US biochemist Casimir Funk (1884–1967) extracts |
|
antiberiberi factor (an amine) from rice husks and coins the term |
|
‘vitamine’ (vital amine; later changed to ‘vitamin’). |
1913 |
US biochemist Elmer McCollum (1879–1967) discovers and names |
|
vitamin A (retinol) and names antiberiberi factor vitamin B. |
1920 |
McCollum names antirachitic factor vitamin D. |
1922 |
US embryologist Herbert Evans (1882–1971) discovers vitamin E |
|
(tocopherol). |
1926 |
German chemist Adolf Windaus (1876–1959) discovers that ergosterol |
|
is converted to vitamin D in the presence of sunlight. |
1931 |
German chemist Paul Karrer (1889–1971) determines the structure of |
|
(and synthesizes) vitamin A. |
1932 |
Hungarian-born US biochemist Albert Szent-Györgyi (1893–1986) and |
|
US biochemist Charles King (1896–1986) independently isolate vitamin |
|
C (ascorbic acid). |
1933 |
Polish-born Swiss chemist Tadeus Reichstein (1897–1996) and British |
|
chemist Walter Haworth (1883–1950) independently synthesize |
|
vitamin C. |
|
US chemist Roger Williams (1893–1988) discovers the B vitamin panto- |
|
thenic acid. |
1934 |
Danish biochemist Carl Dam (1895–1976) discovers vitamin K. |
1935 |
Karrer and Austrian-born German chemist Richard Kuhn (1900–67) |
|
independently synthesize vitamin B2 (riboflavin). |
1937 |
US chemist Robert Williams (1886–1965) synthesizes vitamin B1 |
|
(thamin). |
1938 |
Karrer synthesizes vitamin E. |
|
Kuhn isolates and synthesizes vitamin B6 (pyridoxine). |
1939 |
Dam and Karrer isolate vitamin K. |
1940 |
Szent-Györgyi and US biochemist Vincent Du Vigneaud (1901–78) |
|
discover ‘vitamin H’ (the B vitamin biotin). |
|
Roger Williams determines the structure of pantothenic acid. |
|
US biochemist Edward Doisey synthesizes vitamin K. |
1948 |
US biochemist Karl Folkers (1906– ) isolates vitamin B12 |
|
(cyanocobalamin). |
1956 |
British chemist Dorothy Hodgkin (1910–94) determines the structure of |
|
vitamin B12. |
1971 |
US chemist Robert Woodward (1917–79) and Swiss chemist Albert |
|
Eschenmoser (1925– ) synthesize vitamin B12. |
v

vitamin C |
858 |
serve as components of *coenzymes. Plants and many microorganisms can manufacture B vitamins but dietary sources are essential for most animals. Heat and light tend to destroy B vitamins.
Vitamin B1 (thiamin(e)) is a precursor of the coenzyme thiamine pyrophosphate, which functions in carbohydrate metabolism. DeÜciency leads to *beriberi
in humans and to polyneuritis in birds. Good sources include brewer’s yeast, wheatgerm, beans, peas, and green vegetables.
Vitamin B2 (riboflavin) occurs in green vegetables, yeast, liver, and milk. It is a constituent of the coenzymes *FAD and FMN, which have an important role in the metabolism of all major nutrients as well as in the oxidative phosphorylation reactions of the *electron transport chain. DeÜciency of B2 causes inÛammation of the tongue and lips and mouth sores.
Vitamin B6 (pyridoxine) is widely distributed in cereal grains, yeast, liver, milk, etc. It is a constituent of a coenzyme (pyridoxal phosphate) involved in amino acid metabolism. DeÜciency causes retarded growth, dermatitis, convulsions, and other symptoms.
Vitamin B12 (cyanocobalamin or cobalamin) is manufactured only by microorganisms and natural sources are entirely of animal origin. Liver is especially rich in it. One form of B12 functions as a coenzyme in a number of reactions, including the oxidation of fatty acids and the synthesis of DNA. It also works in conjunction with *folic acid (another B vitamin) in the synthesis of the amino acid methionine and it is required for normal production of red blood cells. Vitamin B12 can only be absorbed from the gut in the presence of a glycoprotein called intrinsic factor; lack of this factor or deÜciency of B12 results in pernicious anaemia.
v Other vitamins in the B complex include *nicotinic acid, *pantothenic acid, *biotin, and *lipoic acid. See also choline; inositol.
vitamin C (ascorbic acid) A colourless crystalline water-soluble vitamin found especially in citrus fruits and green vegetables. Most organisms synthesize it from glucose, but humans and other primates and various other species must obtain it
from their diet. It is required for the maintenance of healthy connective tissue; deÜciency leads to *scurvy. Vitamin C is readily destroyed by heat and light.
vitamin D A fat-soluble vitamin occurring in the form of two steroid derivatives: vitamin D2 (ergocalciferol or calciferol), found in yeast; and vitamin D3
(cholecalciferol), which occurs in animals. Vitamin D2 is formed from a steroid by the action of ultraviolet light and D3 is produced by the action of sunlight on a cholesterol derivative in the skin. Fishliver oils are the major dietary source. The active form of vitamin D is manufactured in response to the secretion of *parathyroid hormone, which occurs when blood calcium levels are low. It causes increased uptake of calcium from the gut, which increases the supply of calcium for bone synthesis. Vitamin D deÜciency causes *rickets in growing animals and osteomalacia in mature animals. Both conditions are characterized by weak deformed bones.
vitamin E (tocopherol) A fat-soluble vitamin consisting of several closely related compounds, deÜciency of which leads to a range of disorders in different species, including muscular dystrophy, liver damage, and infertility. Good sources are cereal grains and green vegetables. Vitamin E prevents the oxidation of unsaturated fatty acids in cell membranes, so maintaining their structure.
vitamin K A fat-soluble vitamin consisting of several related compounds that act as coenzymes in the synthesis of several proteins (including prothrombin) necessary for blood clotting. DeÜciency of vitamin K, which leads to extensive bleeding, is rare because a form of the vitamin is manufactured by intestinal bacteria. Green vegetables and egg yolk are good sources.
vitelline membrane See egg mem-
brane.
vitreous Having a glasslike appearance or structure.
vitreous humour The colourless jelly that Ülls the space between the lens and the retina of the vertebrate eye.
859 |
voltage divider |
vitriol (oil of vitriol) An old name for sulphuric acid. As a result, hydrated iron(II) (ferrous) sulphate was known as green vitriol, and hydrated copper(II) (cupric) sulphate as blue vitriol.
viviparity 1. (in zoology) A form of reproduction in animals in which the developing embryo obtains its nourishment directly from the mother via a *placenta or by other means. Viviparity occurs in some insects and other arthropods, in certain Üshes, amphibians, and reptiles, and in the majority of mammals. Compare oviparity; ovoviviparity. 2. (in botany) a. A form of *asexual reproduction in certain plants, such as the onion, in which the Ûower develops into a budlike structure that forms a new plant when detached from the parent. b. The development of young plants on the inÛorescence of the parent plant, as seen in certain grasses and the spider plant.
vocal cords A pair of elastic membranes that project into the *larynx in air-breathing vertebrates. Vocal sounds are produced when expelled air passing through the larynx vibrates the cords. The pitch of the sound produced depends on the tension of the cords, which is controlled by muscles and cartilages in the larynx.
void A large region of space at the centre of a collection of galaxy superclusters; there are hardly any galaxies in the void itself, indeed there is little evidence of any matter at all. Empty ‘corridors’ connect voids, rather like the holes in a piece of sponge, making them part of the largescale structure of the universe.
volcano A Üssure or vent in the earth’s surface, connected to a magma source in the earth’s interior by a conduit or series of fractures, from which solid, molten, and gaseous material is ejected. The resultant geological structure, also called volcano, can take a number of forms. Central volcanoes, which have a circular vent or number of vents, may be composite volcanoes or stratovolcanoes, comprising alternate layers of tephra (fragmental material including ash) and lava (e.g. Vesuvius, Italy), or (where only solid material is ejected) steep-sided cinder cones. Shield volcanoes are large structures with gentle
slopes (e.g. the islands of Hawaii). Fissure volcanoes are linear fractures in the earth’s surface where Ûuid material is emitted and, on land, spreads over large areas (e.g. volcanoes in Iceland). A number of types of volcanic eruption are recognized: Hawaiian, which are generally quiet with Ûuid lava erupted freely from Üssures or pits, and – with increasing viscocity of magma – Strombolian, Vulcanian, Vesuvian, Plinian, and Peléean, which are more explosive. An exceptionally large form is the supervolcano, in which magma rises to form a vast reservoir in the earth’s crust and builds in pressure over time before erupting in devastating explosions. The last supervolcano to erupt was Toba Caldera in Sumatra,
74 000 years ago. The *caldera of Yellowstone National Park is one of the largest in the world. Volcanoes occur principally along constructive or destructive plate margins (see plate tectonics) but some volcanic activity occurs away from the margins.
volcanology (vulcanology) The scientiÜc study of volcanism, i.e. the processes by which magma and associated gases rise from the earth’s interior and are emitted from the surface, and the resultant structures (see volcano).
volt Symbol V. The SI unit of electric potential, potential difference, or e.m.f. deÜned as the difference of potential between two points on a conductor carrying a constant current of one ampere when the power dissipated between the points is one watt. It is named after Alessandro Volta.
Volta, Alessandro Giuseppe Antonio Anastasio (1745–1827) Italian physicist. In 1774 he began teaching in Como and in that year invented the *electrophorus.
He moved to Pavia University in 1778. In v 1800 he made the *voltaic cell, thus pro-
viding the Ürst practical source of electric current (see also galvani, luigi). The SI unit of voltage is named after him.
voltage Symbol V. An e.m.f. or potential difference expressed in volts.
voltage divider (potential divider; potentiometer) A resistor or a chain of resistors connected in series that can be
voltaic cell |
860 |
tendon
epimysium
R1
bundle of muscle fibres
V
single muscle fibre
R2 |
v |
nucleus |
|
|
myofibril
Voltage divider
tapped at one or more points to obtain a known fraction of the total voltage across the whole resistor or chain. In the illustration, V is the total voltage across the divider and v is required voltage, then
v/V = R2/(R1 + R2).
voltaic cell (galvanic cell) A device that produces an e.m.f. as a result of chemical reactions that take place within it. These reactions occur at the surfaces of two electrodes, each of which dips into an electrolyte. The Ürst voltaic cell, devised by Alessandro Volta (1745–1827), had electrodes of two different metals dipping into brine. See primary cell; secondary
cell.
voltaic pile An early form of battery, devised by Alessandro Volta, consisting of a number of Ûat *voltaic cells joined in series. The liquid electrolyte was absorbed into paper or leather discs.
voltameter (coulometer) 1. An electrolytic cell formerly used to measure quantity of electric charge. The increase in mass (m) of the cathode of the cell as a result of the deposition on it of a metal
vcharge (Q) to be determined from the relationship Q = m/z, where z is the electrochemical equivalent of the metal. 2. Any other type of electrolytic cell used for measurement.
voltmeter An instrument used to measure voltage. *Moving-coil instruments are widely used for this purpose; generally a
galvanometer is used in series with a resistor of high values (sometimes called afrom a solution of its salt enables the
light band
dark band
origin
biceps (flexor) contracted
sarcomere unit containing contractile proteins
 triceps
triceps
(extensor) relaxed
insertion tendon
arm flexed
biceps (flexor) |
triceps |
relaxed |
(extensor) |
|
contracted |
arm extended
Structure and action of a voluntary muscle

861 |
vulva |
multiplier). To measure an alternating potential difference a rectiÜer must be included in the circuit. A moving-iron instrument can be used for either d.c. or a.c. without a rectiÜer. *Cathode-ray oscilloscopes are also used as voltmeters. The electronic digital voltmeter displays the value of the voltage in digits. The input is repeatedly sampled by the voltmeter and the instantaneous values are displayed.
volume Symbol V. The space occupied by a body or mass of Ûuid.
volumetric analysis A method of quantitative analysis using measurement of volumes. For gases, the main technique is in reacting or absorbing gases in graduated containers over mercury, and measuring the volume changes. For liquids, it involves *titrations.
voluntary (in biology) Controlled by conscious thought. See voluntary muscle. Compare involuntary.
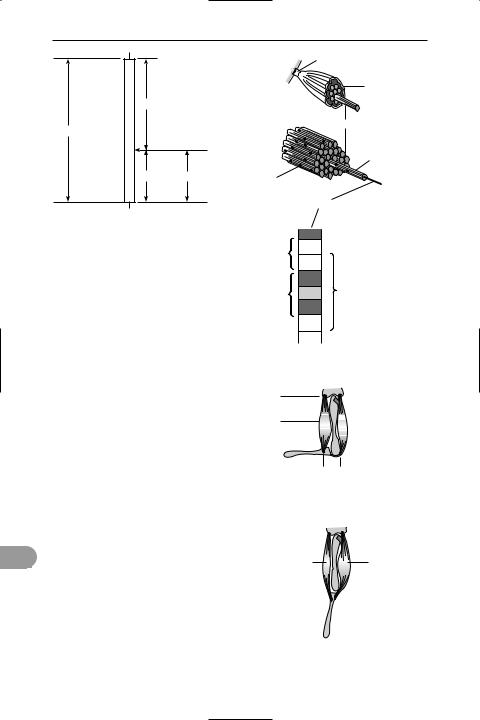
voluntary muscle (skeletal, striped, or striated muscle) Muscle that is under the control of the will and is generally attached to the skeleton. An individual muscle consists of bundles of long muscle Übres, each containing many nuclei, the whole muscle being covered with a strong connective tissue sheath (epimysium) and attached at each end to a bone by inextensible *tendons. Each Übre contains smaller Übres (myoÜbrils) having alternate
light and dark bands, which contain protein Ülaments responsible for the muscle’s contractile ability and give the muscle its typical striped appearance under the microscope. The functional unit of a myoÜbril is the *sarcomere. See illustration.
The end of the muscle that is attached to a nonmoving bone is called the origin of the muscle; the end attached to a moving bone is the insertion. As a muscle contracts it becomes shorter and fatter, moving one bone closer to the other. Since a muscle cannot expand, another muscle (the extensor) is required to move the bone in the opposite direction and stretch the Ürst muscle (known as the Ûexor). The Ûexor and extensor are described as antagonistic muscles. See illustration.
von Laue, Max See laue, max von.
vulcanite (ebonite) A hard black insulating material made by the vulcanization of rubber with a high proportion of sulphur (up to 30%).
vulcanization A process for hardening rubber by heating it with sulphur or sulphur compounds.
vulcanology See volcanology.
vulva The female external genitalia, comprising in women two pairs of Ûeshy folds of tissue, the labia (see labium); the *clitoris; and the vaginal opening.
v

W
Wacker process A process for the manufacture of ethanal by the air oxidation of ethene. A mixture of air and ethene is bubbled through a solution containing palladium(II) chloride and copper(II) chloride. The Pd2+ ions form a complex with the ethene in which the ion is bound to the pi electrons in the C=C bond. This decreases the electron density in the bond, making it susceptible to nucleophilic attack by water molecules. The complex formed breaks down to ethanal and palladium metal. The Cu2+ ions oxidize the palladium back to Pd2+, being reduced to Cu+ ions in the process. The air present oxidizes Cu+ back to Cu2+. Thus the copper(II) and palladium(II) ions effectively act as catalysts in the process, which is now the main source of ethanal and, by further oxidation, ethanoic acid. It can also be applied to other alkenes. It is named after Alexander von Wacker (1846–1922).
Wallace, Alfred Russel (1823–1913) British naturalist, who in 1848 went on an expedition to the Amazon, and in 1854 travelled to the Malay Archipelago. There he noticed the differences between the animals of Asia and Australasia and devised *Wallace’s line, which separates them. This led him to develop a theory of *evolution through *natural selection, which coincided with the views of Charles *Darwin; their theories were presented jointly to the Linnaean Society in 1858.
Wallace’s line An imaginary line that runs between the Indonesian islands of Bali and Lombok and represents the separation of the Australian and Oriental faunas. It was proposed by Alfred Russel Wallace, who had noted that the mammals in SE Asia are different from and more advanced than their Australian counterparts. He suggested this was because the Australian continent had split away from Asia before the better adapted
placental mammals evolved in Asia. Hence the isolated Australian marsupials and monotremes were able to thrive while those in Asia were driven to extinction by competition from placental mammals. See also zoogeography.
wall effect Any effect resulting from the nature or presence of the inside wall of a container on the system it encloses.
Walton, Ernest See cockcroft, sir john douglas.
warfarin 3-(alpha-acetonylbenzyl)-4- hydroxycoumarin: a synthetic *anticoagulant used both therapeutically in clinical medicine and, in lethal doses, as a rodenticide (see pesticide).
warm-blooded animal See endotherm.
warm front See front.
warning coloration (aposematic coloration) The conspicuous markings of an animal that make it easily recognizable and warn would-be predators that it is a poisonous, foul-tasting, or dangerous species. For example, the yellow-and- black striped abdomen of the wasp warns of its sting. See also mimicry.
washing soda *Sodium carbonate decahydrate, Na2CO3.10H2O.
waste product 1. Any product of metabolism that is not required for further metabolic processes and is therefore excreted from the body. Common waste products include nitrogenous compounds (such as *urea and ammonia), carbon dioxide, and *bile. 2. See radioactive waste.
water A colourless liquid, H2O; r.d. 1.000 (4°C); m.p. 0.000°C; b.p. 100.000°C. In the gas phase water consists of single H2O molecules in which the H–O–H angle is 105°. The structure of liquid water is still controversial; hydrogen bonding of the type H2O…H–O–H imposes a high de-

863 |
water potential |
gree of structure and current models supported by X-ray scattering studies have short-range ordered regions, which are constantly disintegrating and re-forming. This ordering of the liquid state is sufÜcient to make the density of water at about 0°C higher than that of the relatively open-structured ice; the maximum density occurs at 3.98°C. This accounts for the well-known phenomenon of ice Ûoating on water and the contraction of water below ice, a fact of enormous biological signiÜcance for all aquatic organisms.
Ice has nine distinct structural modiÜcations of which ordinary ice, or ice I, has an open structure built of puckered sixmembered rings in which each H2O unit is tetrahedrally surrounded by four other
H2O units.
Because of its angular shape the water molecule has a permanent dipole moment and in addition it is strongly hydrogen bonded and has a high dielectric constant. These properties combine to make water a powerful solvent for both polar and ionic compounds. Species in solution are frequently strongly hydrated and in fact ions frequently written as, for example, Cu2+ are essentially [Cu(H2O)6]2+. Crystalline *hydrates are also common for inorganic substances; polar organic compounds, particularly those with O–H and N–H bonds, also form hydrates.
Pure liquid water is very weakly dissociated into H3O+ and OH– ions by self ionization:
H2O ˆ H+ + OH–
(see ionic product) and consequently any species that increases the concentration of the positive species, H3O+, is acidic and species increasing the concentration of the negative species, OH–, are basic (see acid). The phenomena of ion transport in water and the division of materials into hydrophilic (water loving) and hydrophobic (water hating) substances are central features of almost all biological chemistry. A further property of water that is of fundamental importance to the whole planet is its strong absorption in the infrared range of the spectrum and its transparency to visible and near ultraviolet radiation. This allows solar radiation to reach the earth during hours of daylight but restricts rapid heat loss at night. Thus
atmospheric water prevents violent diurnal oscillations in the earth’s ambient temperature. See also greenhouse effect.
water cycle See hydrological cycle.
water gas A mixture of carbon monoxide and hydrogen produced by passing steam over hot carbon (coke):
H2O(g) + C(s) → CO(g) + H2(g)
The reaction is strongly endothermic but the reaction can be used in conjunction with that for *producer gas for making fuel gas. The main use of water gas before World War II was in producing hydrogen for the *Haber process. Here the above reaction was combined with the water-gas shift reaction to increase the amount of hydrogen:
CO + H2O ˆ CO2 + H2
Most hydrogen for the Haber process is now made from natural gas by steam *reforming.
water glass A viscous colloidal solution of sodium silicates in water, used to make silica gel and as a size and preservative.
water of crystallization Water present in crystalline compounds in deÜnite proportions. Many crystalline salts form hydrates containing 1, 2, 3, or more moles of water per mole of compound, and the water may be held in the crystal in various ways. Thus, the water molecules may simply occupy lattice positions in the crystal, or they may form bonds with the anions or the cations present. In the pentahydrate of copper sulphate (CuSO4. 5H2O), for instance, each copper ion is coordinated to four water molecules through the lone pairs on the oxygen to form the *complex [Cu(H2O)4]2+. Each sulphate ion has one water molecule held by hydrogen bonding. The difference between the two types of bonding is demonstrated by the fact that the pentahydrate
converts to the monohydrate at 100°C and w only becomes anhydrous above 250°C.
Water of constitution is an obsolete term for water combined in a compound (as in a metal hydroxide M(OH)2 regarded as a hydrated oxide MO.H2O).
water potential Symbol Ψ. The difference between the chemical potential of
water softening |
864 |
the water in a biological system and the chemical potential of pure water at the same temperature and pressure. It is manifested as a force acting on water molecules in a solution separated from pure water by a membrane that is permeable to water molecules only. Water potential is measured in kilopascals (kPa). The water potential of pure water is zero; aqueous solutions of increasing concentration have increasingly negative values. Water tends to move from areas of high (less negative) water potential to areas of low (more negative) water potential. *Osmosis in plants is now described in terms of water potential.
water softening See hardness of water.
Watson, James Dewey (1928– ) US biochemist, who moved to the Cavendish Laboratory, Cambridge, in 1951 to study the structure of *DNA. In 1953 he and Francis *Crick announced the now accepted two-stranded helical structure for the DNA molecule. In 1962 they shared the Nobel Prize for physiology or medicine with Maurice Wilkins (1916– ), who with Rosalind Franklin (1920–58) had made X-ray diffraction studies of DNA.
Watson–Crick model The doublestranded twisted ladder-like molecular structure of *DNA as determined by James Watson and Francis Crick at Cambridge, England, in 1953. It is commonly known as the double helix.
watt Symbol W. The SI unit of power, deÜned as a power of one joule per second. In electrical contexts it is equal to the rate of energy transformation by an electric current of one ampere Ûowing through a conductor the ends of which are maintained at a potential difference of one volt. The unit is named after James Watt (1736–1819).
wwattmeter An instrument for measuring the power in watts in an alternatingcurrent electric circuit. In a direct-current circuit, power is usually determined by separate measurements of the voltage and the current.
The electrodynamic wattmeter consists of two coils, one Üxed (current) coil and one movable (potential) coil. The Üxed coil
carries the load current, and the movable coil carries a current proportional to the voltage applied to the measured circuit.
The deÛection of the needle attached to the movable coil indicates the power.
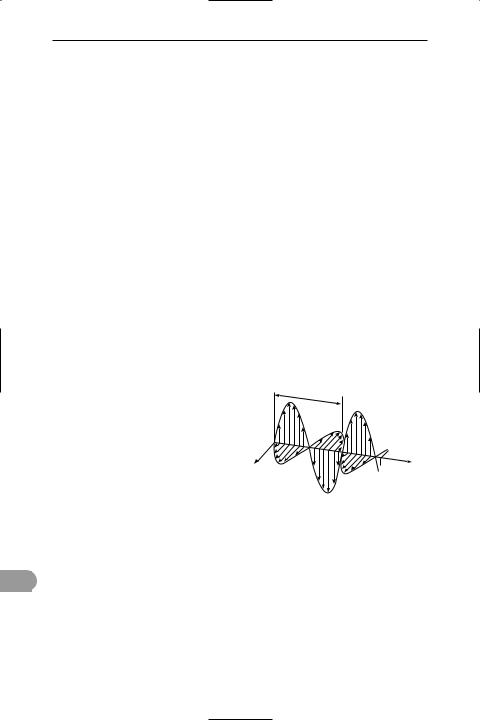
wave A periodic disturbance in a medium or in space. In a travelling wave (or progressive wave) energy is transferred from one place to another by the vibrations (see also stationary wave). In a wave passing over the surface of water, for example, the water rises and falls as the wave passes but the particles of water on average do not move forward with the wave. This is called a transverse wave because the disturbances are at right angles to the direction of propagation. The water surface moves up and down while the waves travel across the surface of the water. Electromagnetic waves (see diagram) are also of this kind, with electric and magnetic Üelds varying in a periodic way at right angles to each other and to the direction of propagation. In sound waves, the air is alternately compressed and rareÜed by displacements in the direction of propagation. Such waves are called longitudinal waves.
electric
field  λ
λ
magnetic |
direction of |
field |
propagation |
Electromagnetic waves
The chief characteristics of a wave are its speed of propagation, its frequency, its wavelength, and its amplitude. The speed of propagation is the distance covered by the wave in unit time. The frequency is the number of complete disturbances (cycles) in unit time, usually expressed in *hertz. The wavelength is the distance in metres between successive points of equal phase in a wave. The amplitude is the maximum difference of the disturbed quantity from its mean value.
Generally, the amplitude (a) is half the

865 |
wave number |
λ
amplitude |
a |
 direction of propagation
direction of propagation
Sine wave
peak-to-peak value. There is a simple relationship between the wavelength (λ) and the frequency ( f ), i.e. λ = c/f, where c is the speed of propagation. The energy transferred by a progressive *sine wave (see diagram) is proportional to a2f 2. See also simple harmonic motion.
wave equation A partial differential equation of the form:
2u = (1/c2)∂2u/∂t2
where
2 = ∂2/∂x2 + ∂2/∂y2 + ∂2/∂z2
is the Laplace operator (see laplace equation). It represents the propagation of a wave, where u is the displacement and c the speed of propagation. See also schrödinger equation.
wave form The shape of a wave or the pattern representing a vibration. It can be illustrated by drawing a graph of the periodically varying quantity against distance for one complete wavelength. See also sine wave.
wavefront A line or surface within a twoor three-dimensional medium
through which waves are passing, being the locus of all adjacent points at which the disturbances are in phase. At large distances from a small source in a uniform medium, the fronts are small parts of a sphere of very large radius and they can be considered as plane. For example, sunlight reaches the earth with plane wavefronts.
wave function A function ψ(x,y,z) appearing in the *Schrödinger equation in *quantum mechanics. The wave function is a mathematical expression involving the coordinates of a particle in space. If the Schrödinger equation can be solved for a particle in a given system (e.g. an electron in an atom) then, depending on
the boundary conditions, the solution is a set of allowed wave functions (eigenfunctions) of the particle, each corresponding to an allowed energy level (eigenvalue).
The physical signiÜcance of the wave function is that the square of its absolute value, |ψ|2, at a point is proportional to the probability of Ünding the particle in a small element of volume, dxdydz, at that point. For an electron in an atom, this gives rise to the idea of atomic and molecular *orbitals.
wave guide A hollow tube through which microwave electromagnetic radiation can be transmitted with relatively little attenuation. They often have a rectangular cross section, but some have a circular cross section. In transverse electric (TE) modes the electric vector of the Üeld has no component in the direction of propagation. In transverse magnetic (TM) modes, the magnetic vector has no such component.
wavelength See wave.
wave mechanics A formulation of *quantum mechanics in which the dual wave–particle nature (see complementarity) of such entities as electrons is described by the *Schrödinger equation. Schrödinger put forward this formulation of quantum mechanics in 1926 and in the same year showed that it was equivalent to *matrix mechanics. Taking into account the *de Broglie wavelength, Schrödinger postulated a wave mechanics that bears the same relation to *Newtonian mechanics as physical optics does to geometrical optics (see optics).
wavemeter A device for measuring the wavelength of electromagnetic radiation. For frequencies up to about 100 MHz a wavemeter consists of a tuned circuit with a suitable indicator to establish when resonance occurs. Usually the tuned circuit includes a variable capacitor cali-
brated to read wavelengths and resonance w is indicated by a current-detecting instru-
ment. At higher frequencies a cavityresonator in a waveguide is often used. The cavity resonator is Ütted with a piston, the position of which determines the resonant frequency of the cavity.
wave number Symbol k. The number
