
A Dictionary of Science
.pdf

|
|
|
transport theory |
832 |
|
|
|
|
|
|
|
|
ing concentration gradient. See active |
Travers, Morris See ramsay, sir |
|
|
|
transport. |
william. |
|
|
|
transport theory The theory of phe- |
Trematoda A class of parasitic Ûat- |
|
|
|
nomena involving the transfer of matter |
worms (see platyhelminthes) comprising |
|
|
|
or heat. The calculation of *transport |
the Ûukes, such as Fasciola (liver Ûuke). |
|
|
|
coefÜcients and inverse transport co- |
Flukes have suckers and hooks to anchor |
|
|
|
efÜcients, such as *conductivity and |
themselves to the host and their body sur- |
|
|
|
*viscosity, is an aim of transport theory. |
face is covered by a protective cuticle. The |
|
|
|
whole life cycle may either occur within |
|
|
|
|
Calculations from Ürst principles in trans- |
|
|
|
|
one host or require one or more interme- |
|
|
|
|
port theory start from *non-equilibrium |
|
|
|
|
diate hosts to transmit the infective eggs |
|
|
|
|
statistical mechanics. Because of the |
|
|
|
|
or larvae. Fasciola hepatica, for example, |
|
|
|
|
difÜculties involved in calculations in non- |
|
|
|
|
undergoes larval development in a land |
|
|
|
|
equilibrium statistical mechanics, trans- |
|
|
|
|
snail (the intermediate host) and infects |
|
|
|
|
port theory uses approximate methods, |
|
|
|
|
sheep (the primary host) when contami- |
|
|
|
|
including the *kinetic theory of gases and |
|
|
|
|
nated grass containing the larvae is swal- |
|
|
|
|
*kinetic equations, such as the *Boltz- |
|
|
|
|
lowed. |
|
|
|
|
mann equation. |
|
|
|
|
triacylglycerol See triglyceride. |
|
|
|
|
transposon (transposable genetic el- |
|
|
|
|
trial-and-error learning See learning. |
|
|
|
|
ement) A mobile genetic element, known |
|
|
|
|
informally as a ‘jumping gene’, that can |
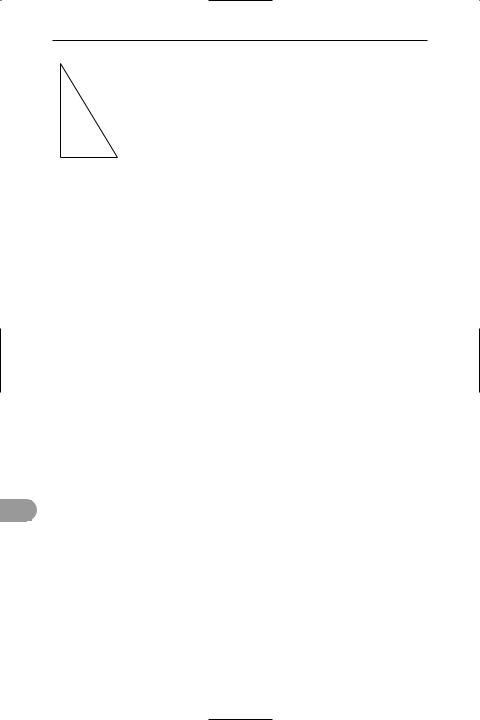
triangle of vectors A triangle con- |
|
|
|
become integrated at many different sites |
structed so that each of its sides repre- |
|
|
|
along a chromosome. The simplest types |
sents one of three coplanar *vectors |
|
|
|
of transposon are known as insertion se- |
acting at a point with no resultant. If the |
|
|
|
quences typically consisting of some |
triangle is completed, with the sides rep- |
|
|
|
700–1500 base pairs and with numerous |
resenting the vectors in both magnitude |
|
|
|
short repeated nucleotide sequences at ei- |
and direction, so that there are no gaps |
|
|
|
ther end. Larger and more complex are |
between the sides, then the vectors are in |
|
|
|
the composite transposons, which consist |
equilibrium. If the three vectors are |
|
|
|
of a central portion, possibly containing |
forces, the Ügure is called a triangle of |
|
|
|
functional genes, Ûanked by insertion se- |
forces; if they are velocities, it is a triangle |
|
|
|
quences at either end. Transposons were |
of velocities. |
|
|
|
Ürst discovered by Barbara McClintock in |
Triassic The earliest period of the Meso- |
|
|
|
maize in the 1940s and have since been |
zoic era. It began about 248 million years |
|
|
|
found in other eukaryotes and in bacteria. |
ago, following the Permian, the last pe- |
|
|
|
They can disrupt gene expression or cause |
riod of the Palaeozoic era, and extended |
|
|
|
deletions and inversions, and hence affect |
until about 213 million years ago when it |
|
|
|
both the genotype and phenotype of the |
was succeeded by the Jurassic. It was |
|
|
|
organisms concerned. Most eukaryotic |
named, by F. von Alberti in 1834, after the |
|
t |
|
transposons are *retrotransposons. Trans- |
sequence of three divisions of strata that |
|
|
posons account for a sizable proportion of |
he studied in central Germany – Bunter, |
|
|
|
|
the *repetitive DNA in eukaryotes. |
Muschelkalk, and Keuper. The Triassic |
|
|
|
transuranic elements Elements with |
rocks are frequently difÜcult to distin- |
|
|
|
guish from the underlying Permian strata |
|
|
|
|
an atomic number greater than 92, i.e. el- |
|
|
|
|
and the term New Red Sandstone is often |
|
|
|
|
ements above uranium in the *periodic |
|
|
|
|
applied to rocks of the Permo-Triassic. |
|
|
|
|
table. Most of these elements are unstable |
|
|
|
|
During the period marine animals |
|
|
|
|
and have short half-lives. See also transac- |
diversiÜed: molluscs were the dominant |
|
|
|
tinide elements. |
invertebrates – ammonites were abundant |
|
|
|
transverse wave See wave. |
and bivalves replaced the declining bra- |
|
|
|
chiopods. Reptiles were the dominant ver- |
|
|
|
|
travelling wave See wave. |
|
|
|
|
tebrates and included turtles, phytosaurs, |
|
|
|
|
travelling-wave accelerator See lin- |
dinosaurs, and the marine ichthyosaurs. |
|
|
|
|
|
|
|
|
ear accelerator. |
triatomic molecule A molecule |

 e
e A b
A b