A Dictionary of Science
.pdf

749 |
|
|
|
|
|
|
|
sinusoidal oscillator |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
y |
|
|
2 |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|||
|
ω |
1 |
|
|
|
|
|
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
r |
|
3 |
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0 |
|
|
4 |
0 |
|
|
4 |
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
t |
|
|
|
|
|
|
y |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5 |
|
|
|
|
|
5 |
|
|
|
|
|
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
|
|
||
Simple harmonic motion |
|
|
|
|
|
|
|
|
|
|
|||
sine wave (sinusoidal wave) |
Any wave- |
apply. It is surrounded by the event hori- |
|
|
|
||||||||
form that has an equation in which one |
|
|
|
zon and so cannot be seen (because no |
|
|
|
||||||
variable is proportional to the sine of the |
light can escape from it). |
|
|
|
|||||||||
other. Such a waveform can be generated |
sinh See hyperbolic functions. |
|
|
|
|||||||||
by an oscillator that executes *simple har- |
|
|
|
||||||||||
sink hole (pot-hole) A saucer-shaped or |
|
|
|
||||||||||
monic motion. |
|
|
|
|
|
|
|
||||||
single bond See chemical bond. |
|
|
|
funnel-shaped depression in a limestone |
|
|
|
||||||
|
|
|
landscape formed when the rock is dis- |
|
|
|
|||||||
single-cell protein (SCP) Protein pro- |
|
|
|
|
|
|
|||||||
|
|
|
solved away by standing water. If a stream |
|
|
|
|||||||
duced by microorganisms, such as bac- |
|
|
|
or river Ûows into the depression and dis- |
|
|
|
||||||
teria, yeasts, and unicellular algae, that is |
appears underground, it is called a swal- |
|
|
|
|||||||||
extracted for use as a component of |
|
|
|
low hole. Some sink holes are formed |
|
|
|
||||||
human and animal foods. |
|
|
|
|
when the roof of a cave collapses. |
|
|
|
|||||
single circulation The type of circula- |
sinoatrial node |
See pacemaker. |
|
|
|
||||||||
tory system that occurs in Üshes, in which |
sintered glass Porous glass made by |
|
|
|
|||||||||
the blood passes only once through the |
|
|
|
|
|
|
|||||||
|
|
|
sintering powdered glass, used for Ültra- |
|
|
|
|||||||
heart in each complete circuit of the body |
|
|
|
||||||||||
tion of precipitates in gravimetric analy- |
|
|
|
||||||||||
Compare double circulation. |
|
|
|
|
|
|
|
||||||
|
|
|
|
sis. |
|
|
|
|
|
||||
single nucleotide polymorphism |
|
|
|
|
|
|
|
|
|||||
|
|
|
sintering The process of heating and |
|
|
|
|||||||
(SNP) A variation in the base sequence oc- |
|
|
|
||||||||||
compacting a powdered material at a tem- |
|
|
|
||||||||||
curring at any given single position in the |
|
|
|
||||||||||
perature below its melting point in order |
|
|
|
||||||||||
genome (for example A instead of C) that |
|
|
|
||||||||||
is found in more than 1% of the popula- |
|
|
|
to weld the particles together into a single |
|
s |
|
||||||
|
|
|
rigid shape. Materials commonly sintered |
|
|
||||||||
tion. It thus differs from a *point muta- |
|
|
|
include metals and alloys, glass, and ce- |
|
|
|
||||||
tion only in its greater frequency. SNPs |
|
|
|
|
|
|
|||||||
|
|
|
ramic oxides. Sintered magnetic ma- |
|
|
|
|||||||
can be found in all parts of the genome, |
|
|
|
|
|
|
|||||||
|
|
|
terials, cooled in a magnetic Üeld, make |
|
|
|
|||||||
including structural genes, regulatory re- |
|
|
|
||||||||||
especially retentive permanent magnets. |
|
|
|
||||||||||
gions, and noncoding ‘junk’ DNA. In the |
|
|
|
||||||||||
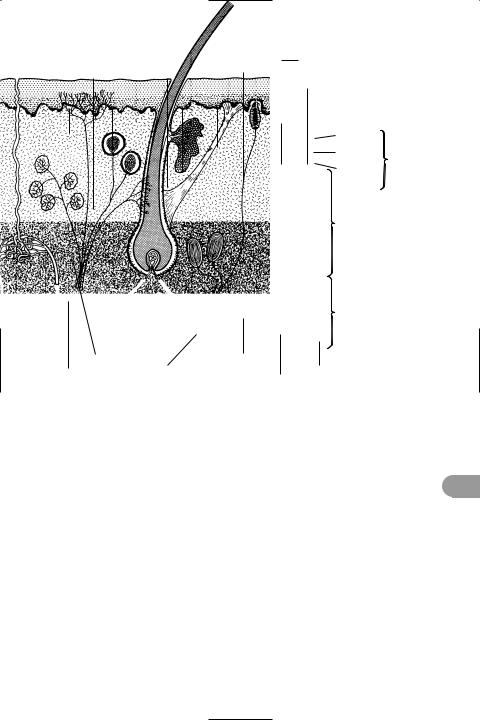
sinus A saclike cavity or organ in an ani- |
|
|
|
||||||||||
human genome overall, many millions of |
|
|
|
||||||||||
SNPs are thought to occur, making them |
mal, e.g. the sinus venosus in the heart of |
|
|
|
|||||||||
exceptionally useful as *molecular mark- |
lower vertebrates. |
|
|
|
|
|
|||||||
ers. Some are known to be linked to dis- |
sinusoid A tiny blood vessel or blood- |
|
|
|
|||||||||
ease-causing alleles. |
|
|
|
|
|
|
|
||||||
|
|
|
|
Ülled space in an organ. Sinusoids replace |
|
|
|
||||||
single-sideband modulation See am- |
|
|
|
||||||||||
capillaries in certain organs, notably the |
|
|
|
||||||||||
plitude modulation. |
|
|
|
|
liver; they allow more direct contact be- |
|
|
|
|||||
singularity An inÜnitely dense point |
|
|
|
tween the blood and the tissue it is sup- |
|
|
|
||||||
|
|
|
plying. |
|
|
|
|
|
|||||
located at the centre of a *black hole, |
|
|
|
|
|
|
|
|
|||||
|
|
|
sinusoidal oscillator See oscillator. |
|
|
|
|||||||
where the laws of physics no longer |
|
|
|
|
|
|
|||||||