

Supplement F2: The Chemistry of Amino, Nitroso, Nitro and Related Groups.
Edited by Saul Patai Copyright 1996 John Wiley & Sons, Ltd.
ISBN: 0-471-95171-4
CHAPTER 15
Photochemistry of amines and amino compounds
TONG-ING HO
Chemistry Department, National Taiwan University, Roosevelt Road Section 4, Taipei, Taiwan (ROC)
Fax: 886-2-363-6359; e-mail: HALL@CHEM50.CH.NTU.EDU.TW
and |
|
YUAN L. CHOW |
|
Department of Chemistry, Simon Fraser University, Burnaby, |
|
British Columbia V5A 1S6, Canada |
|
Fax: (604)291-3765; e-mail: YCHOW@SFU.CA |
|
I. INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
684 |
II. PHOTOCHEMISTRY INVOLVING TERTIARY AMINES . . . . . . . . . . |
685 |
A. With trans-Stilbene . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
685 |
B. With Styrenes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
686 |
C. With ˛,ˇ-Unsaturated Carbonyl Compounds . . . . . . . . . . . . . . . . . . |
687 |
D. With Other Oxidants . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
692 |
E. Tertiary Amines as Donors in Intramolecular |
|
Charge Transfer Interaction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
693 |
III. PHOTOCHEMISTRY WITH SECONDARY AMINES . . . . . . . . . . . . . |
698 |
A. With Excited State trans-Stilbene . . . . . . . . . . . . . . . . . . . . . . . . . |
698 |
B. With Excited Styrenes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
700 |
C. With Other Excited Systems . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
702 |
IV. PHOTOCHEMISTRY INVOLVING PRIMARY AMINES |
|
AND AMMONIA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
704 |
V. PHOTOCHEMISTRY OF IMINE AND IMINIUM SALTS . . . . . . . . . . |
710 |
A. Photoinduced Electron Transfer Chemistry of Iminium Salts . . . . . . . |
710 |
B. The Aza-di- -methane Rearrangement . . . . . . . . . . . . . . . . . . . . . . |
715 |
C. Photochemistry of Azirines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
717 |
D. Stilbene-type Photocyclizations . . . . . . . . . . . . . . . . . . . . . . . . . . . |
717 |
E. Other Reactions of Imines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
720 |
683
684 |
Tong-Ing Ho and Yuan L. Chow |
|
VI. PHOTOCHEMISTRY OF AMIDES AND IMIDES . . . . . . . . . . . . . . . |
722 |
|
A. Amides . . . . . . |
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
722 |
B. Imides . . . . . . . . |
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
730 |
VII. REFERENCES . . . . |
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
740 |
I. INTRODUCTION
Perhaps the most important reaction for the amine is their ability to donate electrons. Since amines generally have low oxidation potentials, they are good electron donors in
their ground state, and the donor ability is further enhanced by photoexcitation. The chemical consequence of this single electron transfer (SET) is the generation of the amine radical cations (aminium radicals) and an earlier review on the aminium radicals is available1.
The SET between amine and acceptor may be enhanced by photoexcitation and may lead to the formation of exciplexes2 or molecular complex with charge transfer character3. The photochemistry between aromatic acceptors and amines via the exciplexes has been discussed earlier (Scheme 1)4.
|
CH3 |
hν |
NEt2 |
|
|
Et3 N |
|
H CH2 N(CH3 )2
hν
(CH3 )3 N
hν
(CH3 )3 N
CH2 N(CH3 )2
SCHEME 1
The importance of tertiary amines in the photochemically induced electron transfer reactions has also been addressed5. Direct irradiation of aromatic or aliphatic amines often leads to the scission of C N, N H or C H bonds that lead to the subsequent chemical reactions by radical pathways6. In this section, photochemical reactions of amines reported since 1978 will be considered with emphasis on photoinduced electron transfer. Photochemical reactions of inorganic and organometallic compounds will not be included unless photochemistry of amine moieties is the primary interest.
15. Photochemistry of amines and amino compounds |
685 |
II. PHOTOCHEMISTRY INVOLVING TERTIARY AMINES
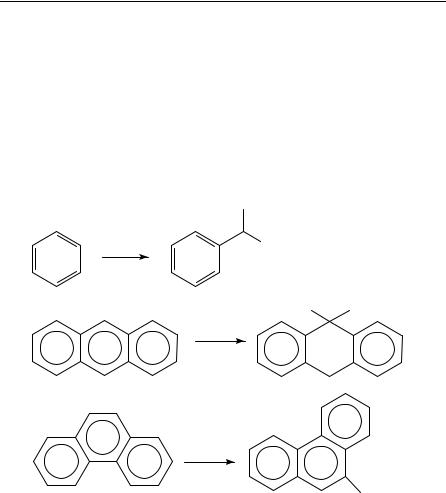
Tertiary amines are known to react with excited aromatic compounds such as benzene7, trans-stilbene8, naphthalene9, anthracene10, phenanthrene11 and cyanoarenes12. The addition of an amine ˛-C H bond to the aromatic compounds is interpreted as the consequence of consecutive electron transfer and proton transfer13 processes that involve several reactive intermediates including exciplexes2 and contact ion radical pairs (CIRP)13 15 from mechanistic considerations17 19. The photoreaction of trans-stilbene (t-S) and a tertiary amine can serve as a model16 to illustrate the multistep process (Scheme 2).
Ph |
H |
|
|
|
|
non- polar |
|
||
|
+ R |
N |
hν |
(t-S− /R N+ |
) |
emission |
|||
|
|
|
|||||||
|
|
|
|
||||||
|
3 |
|
|
3 |
|
solvent |
|
||
H |
Ph |
|
|
exciplex |
|
|
|||
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|||
|
H+ |
|
|
|
|
|
|
|
|
|
|
polar solvent |
|
|
|
|
|
||
|
|
|
|
Ph |
|
|
Ph |
Ph |
|
Ph |
|
|
|
|
|
Ph |
|
||
|
|
|
|
|
|
|
|||
CNR2 |
|
|
|
|
+ |
|
+ |
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
NR2 |
|
|
|
|
Ph |
|
Ph |
|
C |
Ph |
|
Ph |
Ph |
|
(4) radicalpair |
|
|
|
(1) |
|
(2) |
|
|
(3) |
|
|
|
|
SCHEME 2 |
|
|
|
|
|
A. With trans-Stilbene
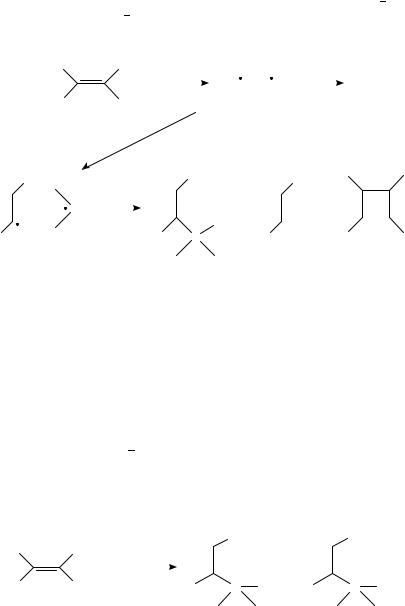
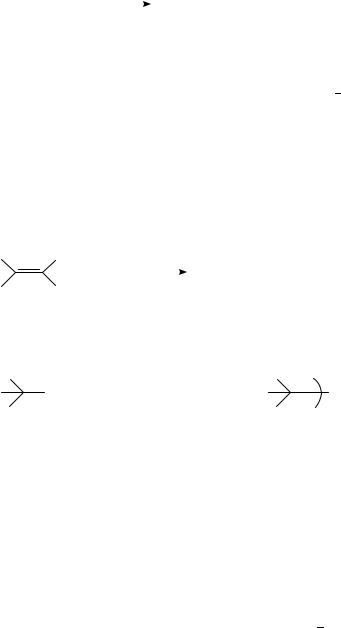
Quenching of t-SŁ by a tertiary aliphatic amine in non-polar solvents yields a fluorescent exciplex whose intensity and lifetime decreased with the increase of solvent polarity; preparatively, products 1, 2 and 3 also increase concurrently. The results have been interpreted as the increasing participation of proton transfer with increasing solvent polarity within the exciplex to give radical pair 4, which is the direct precursor for the observed chemical products. The random proton transfer from the aminium cations (R3NCž ) to the stilbene anion radicals (t-S ž ) practically determines the product pattern20 (equation 1). However, the deprotonation of sterically hindered non-symmetrical tertiary amine cation radicals is regioselective13,21 23 as shown in equation 2. Singlet trans-stilbene reacts with ethyldiisopropylamine by the mechanism to give 5 predominantly (equation 2). It also reacts with amines of structure Me2NCH2R (R D CHDCH2, CO2Et, C CCH3 and C CH) in hexane solution to yield one single adduct PhCH2CHPhCHRNMe2 in each case without involving the methyl group reaction21.
|
|
|
|
|
Ar |
|
Ph |
Ph |
H |
|
hν |
|
|
|
|
|
+ |
R3 N |
|
+ |
(1) |
||
|
|
|
|
||||
H |
Ar |
|
|
Ph |
C |
NR2 Ar |
|
|
|
C NR2 |
|||||
Ar = p-XC6 H4 −
686 |
|
Tong-Ing Ho and Yuan L. Chow |
|
|
|||
t-S + |
|
|
PhCH2 CHPh |
|
PhCH2 CHPh |
||
EtN(i-Pr)2 |
|
|
|
+ |
|
(2) |
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
CH3 CHN(i-Pr)2 |
|
(CH3 )2 CN(i-Pr)C2 H5 |
||
|
|
|
|
|
|
||
|
(5) 92% |
|
(6) 8% |
||||
Photoinduced intramolecular interaction of t-S and tertiary amine moieties linked with a polymethylene chain has also been studied24. The photoexcitation of trans-stilbene in which a tertiary amine is attached to the ortho position with a (CH2)1 3 linker leads to fluorescent exciplexes by intramolecular electron transfer, and results in no more than trans-cis isomerization. The failure to give adducts from the intramolecular exciplexes could arise from the unfavourable exciplex geometry to undergo the necessary bond formation.
B. With Styrenes
Irradiation of styrenes in the presence of tertiary aliphatic amines resulted in the formation of adducts in fair to poor yield25. Excited styrene7 reacted with triethylamine to yield diastereomeric adducts 12, 1-phenylethane 16 and 2,3-diphenyl butane 1926 (equation 3).
R |
R′ |
hν |
|
|
|
|
|
+ N(CH2 R′′′)3 |
|
|
|
|
|
|
|
|
|
|
|
|
Ph |
R′′ |
|
|
|
|
|
(7) R = R′ = R′′ = H |
(10) |
R′′′ = Me |
|
|
||
(8) R = R′′ = H; R′ = Me |
(11) |
R′′′ = H |
|
|
||
(9) R = Me; R′ = R′′ = H |
|
|
|
|
|
|
CHR′ R′′ |
|
|
|
CHR′ R′′ |
(3) |
|
|
|
|
|
|||
Ph |
CHR′′′ N(CH2 R′′′ )2 + |
PhCHRCHR′R′′ + Ph |
|
|||
R |
|
|
|
|
2 |
|
|
|
|
|
R |
|
|
(12) |
R = R′ = R′′ = H; R′′′ = Me |
(16) |
R = R′ = R′′ = H |
(19) R = R′ = R′′ = H |
|
|
(13) |
R = R′′ = R′′′ = H; R′ = Me |
(17) |
R = R′′ = H; R′ = Me (20) R = R′′ = H; R′ = Me |
|||
(14) |
R = R′′ = H; R′ = R′′′ = Me |
(18) |
R = Me; R′ = R′′ = H (21) R = Me; R′ = R′′ = H |
|||
(15) |
R = R′′′ = Me; R′ = R′′ = H |
|
|
|
|
|
The total product quantum yield is higher in acetonitrile than in hexane solution (0.34 vs 0.07); the adduct yield is lower in acetonitrile (9% vs 28% of total product).
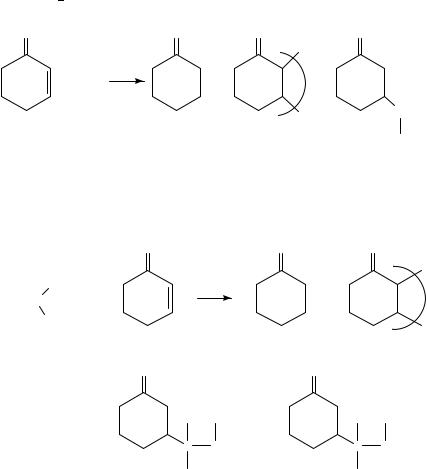
Irradiation of 8 with trimethylamine 11 yields the regiospecific adducts 13, 17 and 2027. The reaction of 8 with triethylamine 10 in either hexane or acetonitrile solution (>290 nm) results in the formation of a single adduct 14 (24% yield) accompanied by comparable amounts of 17 (15%) and also 20 (78%)28 (equation 3). Regiospecific addition of triethylamine to ˛-methylstyrene 9 is also observed to give 15 (32%), 18 (11%) and 21 (8%)26 (equation 3).
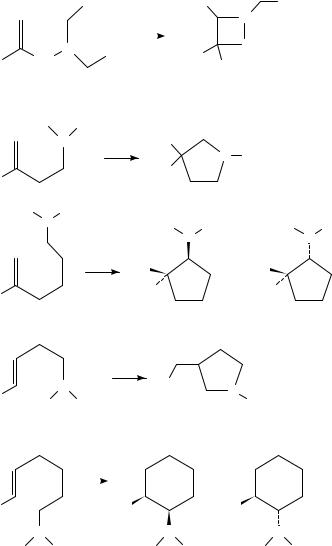
Intramolecular photoaddition of tertiary amine and styrene moieties has been extensively studied by Aoyama29 and Lewis’ group28,30,31 (equations 4 8). Equations 4 and 5 show that if the intramolecular additions result in the formation of a fiveor sixmembered ring, the product yields are excellent. Highly regioselective intramolecular proton transfer is proposed to occur via least motion pathways from the lowest energy
15. Photochemistry of amines and amino compounds |
687 |
folded conformations of the singlet exciplex intermediates in non-polar solvents31.
Ph
|
|
|
|
hν |
|
|
|
|
N |
Ph |
|||
Ph |
(CH2 )n |
|||||
|
|
|
||||
|
n = 2,3 |
|
|
|
|
|
|
CH3 |
CH3 |
||||
|
|
N |
|
CH3 |
||
|
|
|
hν |
|||
Ph |
|
|
|
Ph |
||
|
|
|
|
|
||
|
CH3 |
CH3 |
||||
|
N |
|
|
CH3 |
||
|
|
|
|
|||
|
|
|
hν CH3 |
|||
Ph |
|
|
|
Ph |
||
|
|
|
|
|
||
|
|
|
hν |
|||
Ph |
|
N |
|
Ph |
||
CH3 |
|
CH3 |
||||
|
|
|||||
|
hν |
|
Ph |
|
Ph |
|
||
N |
|
N |
CH3 CH3 |
|
CH3 |
Ph |
Ph |
N |
|
(CH2 )n |
(4) |
Me |
|
Ph |
|
N CH3 (5)
CH3 |
CH3 |
CH3 |
N |
|
N |
+ |
CH3 |
(6) |
|
||
|
Ph |
|
|
4:1 (95%) |
|
N |
CH3 |
(7) |
|
|
|
80% |
|
|
+
|
Ph |
|
(8) |
|
N |
CH3 |
CH3 CH3 |
8:1 (80%)
C. With a,b-Unsaturated Carbonyl Compounds
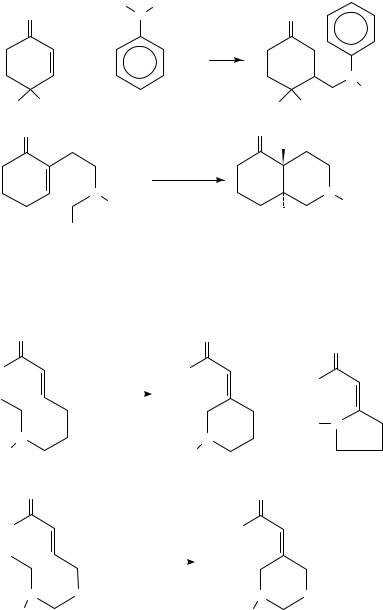
Earlier reviews on the photochemistry of unsatured ketones and amines are available39,40. The photoreactions of ˛,ˇ-unsaturated carbonyl compounds in the presence of amines have been reported to yield 1:1 amine adducts32,33 as well as photoreduction
688 |
Tong-Ing Ho and Yuan L. Chow |
products (equation 9). |
The electron transfer from amine to the triplet enone leads to |
the formation of enone anion and aminium radical pair which has been demonstrated by CIDNP experiments34. Detailed studies by laser flash photolysis concerning the reaction mechanism between triplet enone and amines were carried out by Schuster and coworkers35 38
O |
O |
O |
|
O |
+ Et3 N |
hν |
+ |
+ |
(9) |
|
||||
|
|
|
|
CHNEt2 |
|
|
|
2 |
CH3 |
|
|
|
|
It is shown that the less substituted ˛-C H bond of several tertiary amines added across the olefin bond of 2-cyclohexenone (Scheme 3). In the radical ion pair the proton of the less substituted hydrogen is transferred to the ˛-position of enones that leads to a predominance of products 23, 26, 29 and 32 (about 95% yield).
|
|
O |
|
O |
|
O |
CHR′ R′ |
+ |
|
|
|
+ |
|
RN |
|
|
|
|
||
CHR′′R′′ |
|
|
|
|
|
|
(22,25,28,31) |
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
O |
|
|
O |
|
|
+ |
R′ |
R |
+ |
R′′ |
R |
|
|
C |
NCHR′′R′′ |
|
C |
NCHR′R′ |
|
|
R′ |
|
|
R′′ |
|
|
|
(23,26,29,32) |
|
(24,27,30,33) |
||
22,23,24: R= CH(CH3 )2 ; R′ = H; R′′ = CH3 |
|
|
|
|||
25,26,27: R= CH(CH3 )2 ; R′ = H and CH3 ; R′′ = CH3 |
|
|
|
|||
28,29,30: R=CH3 ; R′ = H and C3 H7; R′′ = H 31, 32,33: R=CH3 ; R′ = H; R′′ = CH3
SCHEME 3
The effect of an ˛-silyl group attached to tertiary amine on 2-cyclohexenone photochemistry has been clarified by Mariano’s group42,43, who illustrates the importance of

15. Photochemistry of amines and amino compounds |
689 |
anions in the proton transfer processes (equation 10).
O |
|
|
O |
|
O |
+ |
Et2 NCH2 SiMe3 |
hν |
|
+ |
|
|
|
NEt2 |
|||
|
|
|
|
NEt2 |
|
R R |
|
|
R R |
SiMe3 |
R R |
R = H or CH3 |
|
|
|
|
|
(34) |
|
(35) |
|||
(10)
Product 34 predominates in the polar aprotic solvent (acetonitrile), while in the polar protic solvent (methanol) products 35 are formed preferentially. The different products are caused by the relative rate of deprotonation against desilylation of the aminium radical, that is in turn governed by the action of enone anion radical in acetonitrile as opposed to that of nucleophilic attack by methanol. In an aprotic, less silophilic solvent (acetonitrile), where the enone anion radical should be a strong base, the proton transfer is favoured and leads to the formation of product 34. In aprotic solvents or when a lithium cation is present, the enone anion radical basicity is reduced by hydrogen bonding or coordination by lithium cation, and the major product is the desilylated 35 (Scheme 4).
O |
|
|
|
O− |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||
+ Et2 NCH2 SiMe3 |
hν |
|
|
|
|
|
+• |
|
H |
|
|
|
|
|
||||
|
|
|
|
|
|
Et2 N |
|
|
CHSiMe3 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
• |
|
|
|
|
|
|
|
|
|
R R |
|
|
|
R R |
|
|
|
|
|
|
|
|
||||||
R = H or CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
H+ |
|
Nu |
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
O |
|
|
|
|
|
|
|
|
O |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
H |
|
|
• |
|
|
|
|
H |
|
|
• |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
Et2 N |
|
CH2 |
||||
|
|
|
• |
Et2 NCHSiMe3 |
|
|
|
|
|
• |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
R R |
|
|
|
|
|
|
|
|
R R |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
34 |
35 |
SCHEME 4

690 Tong-Ing Ho and Yuan L. Chow
The solvent-controlled differential reactivity was also applied to the intramolecular photoaddition of 36 and 37 (equations 11 and 12)45,46.
O |
|
O |
|
O |
|
|
hν |
SiMe3 |
hν |
|
SiMe |
MeCN |
MeOH |
|
|
3 |
|
|
|
|
N |
|
N |
N |
|
Me |
|
Me |
Me |
|
|
(36) |
|
|
|
|
|
|
(11) |
|
|
O |
|
|
O |
|
|
|
O |
|
hν |
|
hν |
(12) |
|
MeCN |
MeOH |
|
|
|
N |
N |
|
N |
SiMe3 |
|
SiMe3 |
|
|
Me |
Me |
|
Me |
|
|
|
|||
(37)
Substituent effects on the ˇ-(aminoethyl)cyclohexenone photochemistry were carried out to study the relative kinetic acidities of the tertiary aminium radical47. The ease of the methylene hydrogen to be removed as HC increased in the order of X D alkyl < Si(CH3)3 < C CH (equation 13).
O
O
|
|
|
hν |
|
R1 |
|
|
|
|
CH2 R1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
N CH R2 |
(13) |
|
|
|
|
|
|
|
||
|
|
|
|
2 |
|||
|
CH2 R2 |
|
|
|
|
|
|
(38) R1 = H, R2 = CH3 |
(41) |
R1 = CH3 , R2 = H (34% in CH3 CN) |
|
||||
(39) R1 = H, R2 = Si(CH3 )3 |
(42) |
R1 = Si(CH3 )3 , R2 = H (76% in CH3 CN) |
|||||
(40) R1 = H, R2 = C |
|
CH |
(43) |
R1 = C |
|
CH, R2 = H (79% in MeOH) |
|
|
|
|
|||||
|
|
|
|||||
|
|
|
|||||
The photosensitized electron transfer by 9,10-dicyanoanthracene (DCA) has been shown to initiate the addition of the ˛-silyl amine 44 to 4,4-dimethylcyclohexenone47 (equation 14). Intramolecular addition of ˛-silyl amine 45 was also shown to be feasible45,46 (equation 15). The primary step is electron transfer to give the aminium
15. Photochemistry of amines and amino compounds |
691 |
radical (44Cž ) and (DCA ž ) anion radical; it is interpreted to be followed by the attack of 44ž on the enone after desilylation.
O |
|
Me |
CH2 SiMe3 |
O |
|
|
N |
|
|
||
|
|
+ |
hν |
|
(14) |
|
|
DCA |
|
N |
|
|
|
|
|
|
|
Me |
Me |
|
|
|
Me |
(44) |
Me |
Me |
|
||
|
|
|
|
|
|
O |
|
|
O |
H |
|
|
|
|
|
|
|
|
|
|
hν, DCA |
|
|
|
|
N |
MeCN (89%) |
N |
(15) |
|
|
CH2 Ph |
|||
|
|
CH2 Ph |
|
H |
|
|
|
|
|
|
|
|
|
SiMe3 |
|
|
|
(45)
In the DCA-sensitized reaction of silyl amino esters 46 (equation 16) the formation of pyrrolidines 48 must be obtained through a desilylmethylation. This process can be prevented by attaching an electron-withdrawing group to the amine that obviously reduces its oxidation potential (equation 17)48.
O |
|
|
|
|
|
O |
O |
|
|
|
|
|
|
|
|
MeO |
|
|
|
MeO |
MeO |
||
|
|
hν, DCA |
|
|
|||
Me3 Si |
|
|
|
+ |
|||
|
|
|
|
|
|
||
|
MeCN−MeOH |
|
|
(16) |
|||
|
|
|
|
||||
|
|
|
|
|
|
|
R N |
N |
|
|
|
|
|
N |
|
R |
|
|
|
|
R |
|
|
(46) |
|
|
|
|
(47) |
(48) |
|
|
|
|
|
|
|||
O |
|
|
|
|
|
O |
|
Y |
|
|
|
|
|
Y |
|
Me3 Si |
|
|
hν, DCA |
|
|
|
|
|
|
|
MeCN−MeOH |
|
|
(17) |
|
N |
(CH2 ) |
|
N |
(CH2 ) |
|||
|
|
n |
|
|
n |
||
PhCH2 O2 C |
|
|
|
|
|
PhCH2 O2 C |
|
Y = Me, OMe
n = 0, 1
692 |
Tong-Ing Ho and Yuan L. Chow |
D. With Other Oxidants
By introducing a proper light-absorbing system, amines can be photoexcited to react with a ground state acceptor. For example, N,N-dimethylaniline is photooxidized by molecular oxygen in the presence of iron complex catalysts in acetonitrile to give a mixture of N-methylformanilide, 4,40-methylenebis(N,N0-dimethylaniline) and N-methylaniline (equation 18)49. The amine N-dealkylation is usually used as a model for enzymatic and cytochrome P-450 oxidation reactions. The potential relationships between the photochemistry of flavin oxidation of amines and monoamine oxidases (MAO) has also been considered50. Anthraquinone fluorescence quenching by electron transfer from amines51 and amine oxidation by triplet carbonyl compounds52 were also studied to clarify electron transfer and deprotonation process.
|
hν, Fe complex |
+ |
|
CHO + PhNCH2 |
NMe2 |
||
PhNMe2 |
|
|
PhNHMe |
PhN |
|||
|
|
||||||
|
O2 |
, MeCN |
|
Me |
|
||
|
|
|
|
|
|
Me |
|
|
|
|
|
+ |
Me2 N |
CH2 |
NMe2 |
(18)
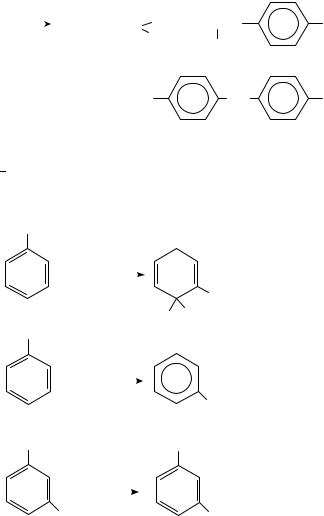
Substituent effects on benzene photochemistry in the presence of amines are described53 in equations 19 21. The ˛-C H of an amine is shown to add photolytically to the 2,5- positions of toluene (equation 19). In contrast, trifluoro-substituted benzene was excited to react with trimethylamine to give 50 and 52 by a side-chain substitution. This chemistry arose from facile defluorination of the anion radicals of 49 and 51.
CH3
+ |
Et3 N |
hν |
(19) |
||||
|
|
|
|||||
|
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|
H CH(Me)NEt2 |
CF3 |
|
|
|
|
|
|
|
+ |
Et3 N |
|
hν |
(20) |
|||
|
|
|
|
||||
|
|
|
|
|
|
|
CF2 CH(Me)NEt2 |
(49) |
|
|
|
|
|
|
(50) |
CF3 |
|
|
|
|
|
|
CF3 |
+ |
Et3 N |
|
|
hν |
(21) |
||
|
|
|
|
||||
CF3 |
|
|
|
|
|
|
CF2 CH(Me)NEt2 |
(51) |
|
|
|
|
|
|
(52) |
