

15. Photochemistry of amines and amino compounds
Norris-type hydrogen abstraction159 (equation 102).
O |
|
|
NHC Me |
NH |
NH2 |
|
|
|
|
MeC 0 |
|
|
hν |
+ |
|
cage |
COMe |
+
hν
CH3 (CH2 )7CH  CH2 + HCONH2 CH3 (CH2 )9 CONH2
CH2 + HCONH2 CH3 (CH2 )9 CONH2
|
(67%) |
|
|
Ph |
hν |
|
|
PhCON(Me)2 + |
PhCOCH2 CH(Ph)2 |
||
|
|||
Ph |
|
|
|
|
hν |
||
PhCONH(CH2 )2 NEt2 |
|
PhCONH2 |
|
|
|||
HO |
|
OH |
|
|
|
|
hν |
+ |
|
HN |
|
|
|
Cl
O
HO
723
NH2
COMe
(96)
NH2
(97)
(98)
(99)
Cl−
HN
O
NH
O
(100)

724 |
Tong-Ing Ho and Yuan L. Chow |
|
O |
O |
O |
NH
O
O hν
I2
O
NH
O
O (70%)
OH
O |
Ph |
N(CHMe2 )2 hν
Ph
O
O
(101)
O
O
Me
Me (102)
N
CHMe2
In addition, recent studies of amides include the 1,3-acyl migration of enamides to obtain the enamines through a photo-Fries like mechanism (equations 103160 and 104161).
|
Me |
Me |
|
Me |
Me |
Ph |
|
|
|||
|
|
hν |
|
NHMe |
|
|
N |
H |
|
||
|
Ph |
|
|||
O |
|
(103) |
|||
Me |
|
|
|
O |
|
|
|
|
|
||
|
|
|
|
|
(100%) |
|
|
|
|
|
O |
|
O |
|
|
|
R |
|
|
|
|
|
|
|
|
|
hν |
|
(104) |
|
|
|
|
|
|
R |
N |
|
|
|
NH2 |
|
|
|
|
|
|
|
H |
|
|
|
|
|
R = Ph, Me |
|
|
|
(85%) |
Enamides 163 undergo photochemical conrotatory six-electron electrocyclic reactions to yield the dihydro intermediate 164, which in turn yields the trans-fused cyclic product 165 (equation 105) by a (1,5)-suprafacial hydrogen shift. Several natural product syntheses like that of benzylisoquinoline and indole type alkaloids can be achieved by this type of photocyclization (equations 106163, 107164, 108165 and 109166).

15. Photochemistry of amines and amino compounds |
725 |
N |
O |
N |
OH |
H |
|
|
|
163 |
|
hν |
|
H |
|
H |
|
|
|
|
|
H |
|
[1,5]H |
|
|
|
H |
|
|
|
shift |
|
|
|
|
|
N |
OH |
N |
O |
H |
|
||
164 |
|
|
|
|
165 |
|
|
|
|
|
|
MeO |
|
|
|
|
|
MeO |
|
|
N |
O |
|
MeO |
|
hν |
N+ |
|
|
||
|
|
MeO |
|
|
|
O |
|
NaBH4
MeO
N
MeO
H
(77%)
H
(105)
O−
O
(106)
O
H
O

726 |
|
Tong-Ing Ho and Yuan L. Chow |
|
|
|
||
|
|
O |
|
− |
|
|
|
|
|
|
|
O |
|
|
|
|
|
CH2 Ph |
|
|
+ CH2 Ph |
|
|
|
|
N |
|
|
N |
|
|
|
|
hν |
|
|
|
|
|
|
|
MeOH |
|
|
|
|
|
R1 |
|
R2 |
1 |
|
R |
2 |
|
|
|
|
R |
|
|
|
|
|
|
|
|
I2 |
|
|
(107) |
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
CH2 Ph |
|
|
|
|
|
|
|
N |
|
|
|
|
|
R1 |
|
R2 |
|
|
|
|
|
|
(88%) |
|
|
|
|
|
O |
|
|
|
|
|
|
|
N |
OMe |
|
|
|
|
|
|
|
|
CO2Me |
|
|
|
|
|
N |
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
hν |
|
|
|
|
|
|
|
|
|
O |
|
(108) |
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
H |
CO2Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(60%) |
|
|
|
|
O |
O |
|
O |
H |
|
|
|
|
O |
|
|
|||
|
|
|
|
|
|
||
|
|
|
|
H |
|
|
|
|
|
N |
|
H |
N |
|
|
|
|
|
|
|
|
||
|
|
Me |
|
|
Me |
(109) |
|
|
|
hν |
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
NaBH4 |
|
|
|
|
|
|
N |
|
|
N |
|
|
|
R |
|
|
|
R |
|
|
|

15. Photochemistry of amines and amino compounds |
727 |
The enamide 166 react with the cation radical of cyclohexadiene, which is generated by sensitized electron transfer with the photoexcited dicyanobenzene (DCB), to generate
a Diels-Alder type adduct (equation 110)167.
+ |
Me |
hν |
|
N |
DCB |
||
|
Ac
(166)
+ 
+ |
Me |
|
N |
||
|
||
|
Ac |
(110)
H Me
H N
Ac
Dienamides such as 167 react by a different reaction pathway168, namely by addition of the amide oxygen to the alkene by a radical addition reaction (equation 111).
hν |
O |
|
|
O |
|
O |
|
|
|
|
|
NH |
R |
N |
R |
N |
|
R |
|||||
|
|
|
|
||
R = Ar, Me |
|
H |
|
|
|
(167) |
|
|
|
|
(111)
Chiral induction to obtain the ˇ-lactam 169 by photolysis of the chiral crystal 168 in the solid state is possible (equation 112).
|
O |
|
OH |
Me |
||
Ph C |
|
Ph |
||||
|
|
|||||
CHMe2 |
hν |
|
|
|
Me |
|
|
|
|
||||
|
|
|
|
|
|
|
C |
N |
|
|
N |
(112) |
|
|
|
|||||
O |
CHMe2 |
O |
CHMe |
|||
|
|
|
|
|
2 |
|
(168) |
(−)-crystal |
|
(−)-(169) |
|||
The photoreaction of N-(12-dodecanoic acid)-benzoylformamide 170 in solution and in ˇ-cyclodextrin or carboxymethylamylose complexes indicated that major products are those of hydrolysis to mandelamide 171 and to the corresponding aldehyde 172 (equation 113)169.

728 Tong-Ing Ho and Yuan L. Chow
|
O |
|
|
OH |
|
|
H |
|
|
|
|
|
N |
hν |
Ph |
CH |
NH2 |
Ph |
CH2 (CH2 )10 CO2 H |
C |
|
||
|
|
||||
|
O |
|
|
O |
|
|
(170) |
|
|
(171) |
(113) |
O
+ H C (CH2 )10 CO2 H
(172)
The intramolecular photochemistry of the vinylogous amide 173 in tert-butyl alcohol yielded a retro-Mannich type reaction product 174 (equation 114)170.
O |
O |
|
H |
|
hν |
|
(CH2 )2 |
|
N |
|
H |
|
N |
(173) |
(174) |
|
(114) |
Compared to amides, the photochemistry of thioamides was less studied. The 2- substituted aryl thioamide 175 undergoes photocyclization to quinoline derivatives (equation 115)171.
|
R2 |
|
R2 |
|
|
hν |
(115) |
|
|
|
|
N C |
R1 |
N |
R1 |
|
|||
H |
|
|
|
S
(175)
Thioamides 176 react photochemically with 2,3-dimethyl-2-butene in the absence of oxygen to give ketones (equation 116)172. In the absence of oxygen, the photoproducts of 176 include nitriles, 1,2,4-thiadiazole and isothiazoline (equation 117).

15. Photochemistry of amines and amino compounds
S |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Me |
|||
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|
Me |
|
|
|
|
S |
|
Me |
||||||||||||||||||
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
hν |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
C |
|
|
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
ArC |
|
|
NH2 |
|
Me |
|
|
|
|
|
|
|
|
|
|
|
N2 |
|
|
Ar |
|
|
|
|
|
|
|
|
|||||||||||
(176) |
|
|
|
|
|
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|
|
|
Me |
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH2 |
Me |
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
hν |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH |
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
Ar |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
C |
|
|
|
CHMe2 |
|
|
|
|
Ar |
|
C |
|
|
CHMe2 |
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
|
|
|
S |
|
hν |
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
− |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
Ar |
|
|
C |
|
N |
|
S |
|
ArC |
|
|
N + S |
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
O2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Ar |
|
C NH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
ArCN |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
Ar |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ar |
Me |
|
|
||||||||||
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Me |
|
|
||||||||
|
|
|
|
|
|
N |
|
Ar |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
Me |
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
S |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S |
|
Me |
|
|
|||||
729
(116)
(117)
Primary thioamides undergo photochemical hydrogen sulphide extrusion in the absence of oxygen (equation 118)173.
S |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
hν |
|
|
N + |
|
(118) |
RC |
NH2 |
RC |
|
H2 S |
||||||
|
|
|||||||||
|
N2 |
|
|
|||||||
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
Finally the effects of conformation, hydrogen bonding and Lewis acids on the intramolecular electron transfer, spectroscopy and photochemistry of amides were recently
studied174 176 (equations 119 |
121). |
|
|
|
O |
|
|
|
|
R3 |
R1 |
|
N |
|
|
|
hν |
|
|
|
|
|
|
R1 |
R2 |
X |
R2 |
X |
|
N O |
|
|
|
|
R3 |
X = H , CF3 , OMe
R1 , R2 , R3 = H , Me
(119)

730 |
Tong-Ing Ho and Yuan L. Chow |
|
|
|
Me |
|
H |
|
|
|
|
|
|
|
N |
|
Me |
|
O |
N |
N |
N |
|
|
H |
Me |
|
|
O |
|
Me |
|
|
|
(120) |
|
O |
O |
|
|
R |
|
Me |
|
N |
N |
|
|
Me |
R |
(121) |
|
|
|
R = Me, C4 H9 , CH2 CH2 NMe2 , CH2 CH2 CH2 NMe2 , CH2 CH2 NMePh
The first example of a counterthermodynamic one-way E ! Z photoisomerization based upon intramolecular hydrogen bonding was reported for the N-methyl-3-(2-pyridyl)- propenamide systems.
B. Imides
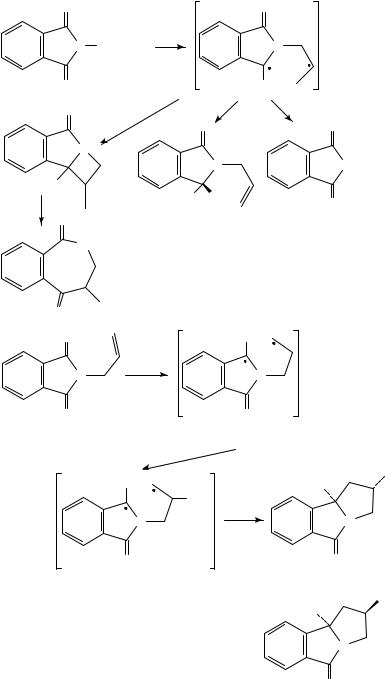
There are several reviews available on the photochemistry of imides177. The photochemistry of N-alkylphthalimides (equation 122) has been extensively studied178. Photochemical cyclization, ˇ-cleavage and hydrogen atom transfer reactions are caused by intramolecular υ-H atom abstraction from the T1 ( Ł ) state of the phthalimide carbonyl group. The reaction is dependent on the solvent used. Benzazepinedione formation is observed in ethanol while ˇ-cleavage and H-transfer occur in acetone and acetonitrile. Both intermolecular and intramolecular electron transfers are important pathways for imides179. For example180, irradiation of N-allylphthalimide in methanol results in intramolecular electron transfer to yield the radical ion pair 177a followed by an anti-Markovnikov addition of the methanol to produce the biradical 177b. The cyclized products 178 and 179 are derived from the biradical intermediate (equation 123). The macrocyclic compound 180 was also synthesized using this methodology180 (equation 124). When a heteroatom such as nitrogen, oxygen or sulphur is introduced into the N-alkyl side chain of the phthalimide, photoinduced electron transfer to generate radical ion pairs becomes feasible181. Proton transfer from the methyl or methylene group adjacent to the heteroatom gives a diradical which will cyclize the products (equation 125). When the substituent is methylthio (MeS) rather than dimethylamino (NMe2), the chemical yield increases and medium to large rings containing 38 atoms can be achieved182.
15. Photochemistry of amines and amino compounds
O |
|
O |
|
|
hν |
N |
|
N (CH2 )2 CH3 |
|||
O |
|
OH |
|
O |
|
|
|
|
O |
O |
|
N |
|
|
|
|
N |
NH |
|
HO |
|
|
|
|
HO H |
O |
|
|
|
||
O |
|
|
|
NH |
|
|
|
O |
|
|
|
O |
|
O− |
|
|
|
+ |
|
N |
hν |
N |
|
MeOH |
|||
|
|
||
O |
|
O |
|
|
|
(177a) |
|
731
(122)
MeOH |
|
|
OH |
|
OMe |
HO |
|
|
OMe |
|
|
N |
N |
(123) |
|
||
O |
O |
|
(177b) |
(178) 41% |
|
|
|
OMe |
|
HO |
|
+ |
N |
|
|
O |
|
|
(179) 41% |
|

732 |
Tong-Ing Ho and Yuan L. Chow |
O
O
N
O (CH2 )n O CH2 CH CHPh
O |
MeOH hν |
|
Ph |
OMe |
(124) |
HO |
O |
|
|
|
|
|
O |
(CH2 )n |
N |
|
O |
|
|
|
O |
|
|
(180) |
60% |
|
O
N (CH2 )nCH2 X CH3
hν
O
− H+
O
N (CH2 )nCH2 +
• |
• |
CH2 |
|
|
|
OH |
CH2 X |
|
|
|
O
N (CH2 )n
CH2
HO CH2 X
(78%; n = 4, X = S)
O
+ •
N (CH2 )nCH2 X CH3
•
O−
O
N (CH2 )nCH• 2 X CH3
•
OH
O
N
(CH2 )n
HO
XMe (6%; n = 4, X = S)
(125)
