

15. Photochemistry of amines and amino compounds |
693 |
The electron transfer reaction of excited benzophenone and trialkylamines has been applied to design photochemical cells54.
N,N-Dimethylaniline is demethylated by excited 3-nitrochlorobenzene55 (equation 22) in which the latter acts as the electron acceptor; subsequent proton transfer and hydrolysis complete the sequence.
NMe2 Cl NHMe
+ |
hν |
(22) |
|
||
|
|
|
|
NO2 |
|
E. Tertiary Amines as Donors in Intramolecular Charge Transfer Interaction
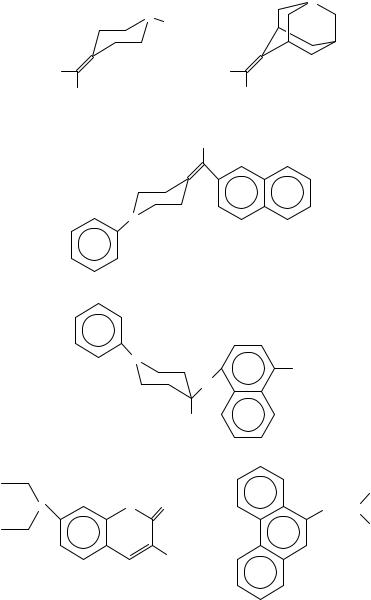
The photophysical aspects of interand intramolecular charge transfer interaction between benzenoid chromophores and tertiary amines continues to attract attention56 with the aim of clarifying the intermediates, e.g. exciplexes or radical ion pairs. There are many intramolecular charge transfer systems published in which tertiary amines are used as donors in the intramolecular charge transfer (ICT), twist intramolecular charge transfer (TICT) and in intramolecular exciplex formation. Typical examples are summarized in Scheme 5. For example, the emission states for compounds 56 ( max D 586 nm, emission maxima in acetonitrile), 57 ( max D 297, toluene), 65, 67, 74 (for n D 2, max D 369 nm,
524 nm in DMSO), 76 ( max D 458 nm in ethyl acetate, max D 486 nm in CH3CN) and 83 are classified as intramolecular exciplex systems, since the excitation is primarily on
the donor or acceptor and the precursor states for exciplexes are locally excited states. The
emission states for compounds 53 ( max D 351 nm, 489 nm, CH3CN), 54, 55, 58, 62, 73 ( max D 486 nm, CHCl3; max D 508 nm in DMSO), 75 ( max D 412 nm, 430 nm, in methylcyclohexane) are classified as TICT states because there is mesomeric interaction
in the ground state and excitation will cause a twist movement accompanied by the change transfer. Compound 53 exhibits two fluorescence bands, the normal b band and the longwave a band. The a band appears only in polar solvents; it grows with the polarity of the solvent and shifts strongly to the red. Compound 77, in which the amine is immobilized
N
N
N
CN |
COOEt |
|
|
|
|
(53)57,62 |
(54)57 |
(55)57 |
SCHEME 5

694 |
Tong-Ing Ho and Yuan L. Chow |
|
|
|
N |
|
|
N |
|
|
N |
N |
N |
|
(56)58 |
(57)59 |
(58)6 0 |
N
(59)61,6 4
NC |
CH CH |
N |
N |
|
(60)63 |
|
(61)65 |
|
CH3 |
|
|
|
|
|
(CH2 )3 |
N |
O |
O |
|
|
(62)66 |
|
(63)67,75 |
CN
CN
N
SCHEME 5 (continued)

15. Photochemistry of amines and amino compounds |
695 |
|
O |
O |
N (CH2 ) O |
C |
COCH |
n |
|
3 |
(n =1, 2, 3, 4, 5, 10)
(64)68
(CH2 )n
(65)69
O
N (CH2 )n N
O
(n =2, 3, 4, 7)
(66)70
(CH2 )n-NR2
Ph
(67)71_ 73,77
SCHEME 5 (continued)
N
Cl
Cl
Cl
Cl
NMe2
(68)74
696 |
Tong-Ing Ho and Yuan L. Chow |
N
N Me
CN |
NC |
CN |
CO2 Et |
(69)76 |
(70)76 |
|
H |
N
(71)78
N
CN
CH2
H
(72)79
N |
O |
O |
(CH2 )nN |
|
CHO
( n =1, 2, 3)
(73)80 |
(74)81 |
SCHEME 5 (continued)

15. Photochemistry of amines and amino compounds |
697 |
O |
N |
|
O
O |
NCH2 |
|
C
N
N
(75)82 |
(76)83 |
(77)84 ,85 |
(SiMe2 )n Me
NC
N
(n =2,3,6 )
(78)86 |
(79)87 |
N O O
N N O O
CHO
N CH3
(80)88
(81)89
N |
( |
) |
NO2 |
|
|
||
|
|
n |
|
( n =1, 2, 3 )
(82)90
SCHEME 5 (continued)

698 |
Tong-Ing Ho and Yuan L. Chow |
|
|
|
Me |
|
N |
N |
Me
(83)98
SCHEME 5 (continued)
in a five-membered ring, emits the b band only. For most compounds, the intramolecular electron transfer is involved in the photoexcitation process even though the extent of electron transfer might not be one hundred percent.
III. PHOTOCHEMISTRY WITH SECONDARY AMINES
The intermolecular photochemical reactions of aryl olefins25,91 and arenes92,93 with secondary aliphatic amines result in the addition of an N H bond to the arenes. The products of the reduction and reductive dimerization of the aryl olefins are also observed. The mechanism8 proposed for the stilbene-secondary amine addition (Scheme 6) involves electron transfer quenching of singlet stilbene (t-S) by a ground-state amine to form a singlet exciplex which undergoes N H transfer to form a radical pair; this may either combine, disproportionate or diffuse apart22. The absence of exciplex fluorescence from singlet stilbene and other arenes with secondary amines may be due to rapid N H transfer which occurs in both non-polar and polar solvents94 96.
|
|
|
+ |
|
|
Ph |
|
|
|
|
|
|
|
||
1t-S* + R2 NH |
|
1 (t-S−/R2 NH) |
|
|
NR2 |
||
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Ph |
|
|
|
|
|
Ph |
|
Ph |
Ph |
Ph |
|
|
|
|
|
|||
|
|
|
|
|
|
+ |
|
|
|
Ph |
NR2 |
Ph |
Ph |
Ph |
|
SCHEME 6
A. With Excited State trans-Stilbene
Irradiation of o-methyl-trans-stilbene with diethylamine in acetonitrile solution results in the formation of two regioisomeric adducts in a 1:1 ratio, together with comparable

15. Photochemistry of amines and amino compounds |
699 |
amounts of reduced stilbene24 (equation 23). Recent focus is on the intramolecular photochemistry of secondary (aminoalkyl) stilbenes24,28,97. Irradiation of trans-2-[(N- methylamino)methyl] stilbene 84 results in the slow formation of several products (equation 24). The major product can be converted to the aldehyde 86 (27% isolated yield). Irradiation of trans-2-[2-(N-methylamino)-ethyl] stilbene 87 results in the formation of N- methyl-2-phenyltetrahydro-3-benzazepine 88 as the only significant product (equation 25).
|
hν |
NEt2 |
|
+ Et2 NH |
|
|
|
|
CH3 CN |
|
|
Me |
|
Me |
|
|
|
|
(23) |
|
NEt2 |
|
|
+ |
|
+ |
|
|
CH3 |
Me |
|
Ph |
|
Ph |
Ph |
H |
hν |
+ |
|
|
(24) |
||
|
|
||
N |
|
N |
O |
|
Me |
Me |
|
|
|
|
H |
(84) |
|
(85) |
(86) |
Ph |
|
|
|
|
|
Ph |
|
|
H |
|
|
H |
hν |
N |
(25) |
|
|||
|
Me |
Me |
|
|
|
|
|
(87) |
(88) |

700 |
Tong-Ing Ho and Yuan L. Chow |
Irradiation |
trans-2-[3-(N-methylamino)propyl] stilbene 89 results in the formation |
of N-methyl-1-benzyltetrahydro-2-benzazepine 90 as the only significant primary photoproduct (equation 26), which in turn undergoes secondary photochemical N- demethylation. The final mixture contains 90 (38%) and 91 (25%) at high (>95%) conversion. Intramolecular photoadditions of these (equations 24 26) secondary (aminoalkyl)-stilbenes are highly regioselective processes24.
Ph |
|
Ph |
Ph |
H |
|
Me |
H |
|
N |
N |
|
H |
|
||
hν |
hν |
|
|
Me |
|
||
|
|
|
|
(89) |
|
(90) |
(91) |
|
|
|
(26) |
B. With Excited Styrenes
Irradiation of styrenes in the presence of secondary aliphatic amines resulted in the regioselective addition of the N H bond to the styrene25. The mechanism proposed for the formation of addition and reduction products from ˇ-methylstyrene and diethylamine is outlined by equation 2799. Electron transfer quenching of singlet styrene by diethylamine followed by regioselective proton transfer to styrene ˇ-C yields a radical pair which combines with the adducts. The escaped ˛-styryl radical can disproportionate or combine. Irradiation of ˛-methylstyrene with diethylamine in deoxygenated hexane solution results in the formation of the regioselective adduct 92 and the reduction products 93 and 94 in approximately equal amounts100 (equation 28).
|
|
hν |
− |
|
+ • |
|
|
|
|
||||
|
+ Et2 NH |
|
|
• |
Et2 NΗ |
|
|
• |
+ Et2 N• |
|
|
||
Ph |
Ph |
Ph |
Ph |
NEt2 |
|||||||||
|
|
|
|
|
|||||||||
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
Ph |
|
|
|
|
|
|
|
+ |
|
+ |
|
|
|||
|
|
|
|
|
|
|
|
|
Ph |
|
Ph |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(27) |
|
|
|
hν |
NEt2 |
+ |
|
|
+ |
|
|
|
|||
|
+ Et2 NH |
|
|
|
|
|
|
Ph |
|||||
Ph |
|
|
|
|
|
Ph |
|
|
|
||||
|
Ph |
|
|
|
Ph |
(28) |
|||||||
(92) |
(93) |
(94) |

15. Photochemistry of amines and amino compounds |
701 |
Intramolecular secondary aminostyrenes 95 97 were also studied100. N-2,2-Trimethyl- 3-phenyl-3-buten-1-amine 95 was irradiated to obtain the elimination product 98 (equation 29). Irradiation of N-methyl-4-phenyl-4-penten-1-amine 96 results in a single product 99 in 80% yield by GC analysis (equation 30). Similarily, irradiation of N-methyl- 5-phenyl-5-hexen-1-amine 97 results in the formation of a single product 100 in 70% yield (equation 31). The photochemistry of the (aminopropyl) indene 101 is also similar (equation 32).
|
H |
Me |
|
|
Me |
|
|
N |
hν |
|
•N |
|
|
|
|
|
|
|
|
|
|
|
|
• |
|
|
(29) |
Ph |
|
|
Ph |
|
Ph |
|
|
(95) |
|
|
|
(98) 50% |
|
|
|
H |
|
|
|
|
|
|
N |
hν |
Ph |
|
|
|
|
|
N |
(30) |
||
Ph |
(CH2 )3 |
|
Me |
|
||
|
|
|
||||
|
|
|
|
|||
|
|
|
|
|
Me |
|
|
(96) |
|
|
|
(99) |
|
|
|
H |
|
|
|
|
|
|
N |
hν |
|
|
|
|
|
|
|
|
|
|
Ph |
(CH2 )4 |
|
Me |
Ph |
N |
(31) |
|
|
|
|
|
Me |
|
|
(97) |
|
|
|
(100) |
|
|
|
HN |
|
|
N |
CH2 Ph |
|
|
CH2 Ph |
hν |
|
(32) |
|
|
|
|
|
|||
|
(101) |
|
|
|
70% |
|
High yields of nitrogen heterocycles have been achieved28,99 by irradiation of several ˇ-[N-methylaminoalkyl] styrenes in which the chain length (n D 1 to 5) (equation 33, n D 1 5) determines the yield, quantum yield and ratio of regioisomeric adducts. The product of addition of nitrogen to the benzylic carbon (b) is exclusive for n D 3, and is predominant for n D 1,2 or 5 (b:a > 10:1 in acetonitrile solution). However, a predominates for n D 4 (a:b D 7:1 in acetonitrile). When n D 1 or 2 the chain length may be too short to allow hydrogen transfer to the ˇ end of the styrene double bond. When the chain is sufficiently long, the intramolecular exciplex would be expected to display chemistry similar to that of intermolecular systems. For intermediate chain lengths (n D 3 or 4) the geometry of the exciplex, as determined by chain folding energies, may determine the regioselectivity of hydrogen transfer. The large isolated yield for n D 2 4 (60 80%) is

702 |
Tong-Ing Ho and Yuan L. Chow |
promising for the synthesis of macrocyclic naturally occuring alkaloids.
(CH2 )n |
Me |
|
(CH2 )n |
(CH2 )n |
|
N |
|
hν |
N |
||
|
|
+ |
|
||
|
|
|
(33) |
||
H |
|
|
Me |
N |
|
Ph |
|
|
Ph |
Ph |
Me |
n = 1−5 |
|
|
(a) |
(b) |
|
C. With Other Excited Systems
The substituent effects on the photochemistry between benzene and secondary aliphatic amines53 were studied. Irradiation of toluene or chlorobenzene with diethylamine results in the formation of mixtures of addition and substitution products (equations 34 and 35). Irradiation of anisole or benzonitrile with diethylamine gives the substitution product N,N-diethylaniline (equations 36 and 37). Irradiation of benzylfluoride with diethylamine results in a side-chain substitution (equation 38). The photoreaction of p-fluorotoluene with diethylamine gives both substitution and reduction products (equation 39).
Me
Me
|
|
|
|
|
|
Me |
|
Et2 NH |
+ |
|
|
+ |
|
|
+ |
hν |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
Me |
|
|
|
H |
NEt2 |
H |
NEt2 |
|
H NEt2 |
NEt2 |
|
Cl |
|
|
|
|
|
|
Cl |
|
|
|
|
|
Cl |
|
|
Et2 NH |
|
+ |
|
|
|
+ |
|
hν |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
NEt2 |
|
H |
NEt2 |
H |
NEt2 |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cl |
|
+ |
+ |
|
|
|
+ |
|
|
|
|
Cl |
NEt2 |
|
|
|
|
CH |
|
H |
|
H |
NEt2 |
||
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
Me |
NHEt |
|
|
|
|
|
|
|
Et Et |
|
|
|
|
|
|
OMe |
N |
|
|
|
|
|
|
Et2 NH
hν
Me
(34)
(35)
(36)
