

Supplement F2: The Chemistry of Amino, Nitroso, Nitro and Related Groups.
Edited by Saul Patai Copyright 1996 John Wiley & Sons, Ltd.
ISBN: 0-471-95171-4
CHAPTER 18
The electrochemistry of nitro, nitroso, and related compounds
ALBERT J. FRY |
|
Wesleyan University, Middletown, Connecticut, USA |
|
Fax: (860)685-2211; e-mail: AFRY@WESLEYAN.EDU |
|
I. INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
837 |
II. GENERAL CONSIDERATIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . |
838 |
A. Mechanism of Electrochemical Reduction . . . . . . . . . . . . . . . . . . . |
838 |
1. Dependence of proton activity of medium . . . . . . . . . . . . . . . . . |
838 |
2. Other medium effects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
843 |
III. SUBSTITUTED NITROAROMATICS . . . . . . . . . . . . . . . . . . . . . . . |
845 |
IV. AROMATIC DINITRO COMPOUNDS . . . . . . . . . . . . . . . . . . . . . . . |
847 |
V. ALIPHATIC NITRO COMPOUNDS . . . . . . . . . . . . . . . . . . . . . . . . |
849 |
VI. RELATED PROCESSES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
851 |
A. Nitro Compounds as Electrogenerated Bases . . . . . . . . . . . . . . . . . |
851 |
B. Nitro Compounds as Protecting Groups . . . . . . . . . . . . . . . . . . . . . |
852 |
VII. NITROSO COMPOUNDS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
854 |
VIII. ACKNOWLEDGMENTS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
854 |
IX. REFERENCES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
854 |
I. INTRODUCTION
Nitro compounds have been popular subjects for investigation from the earliest days of organic electrochemistry. The reasons for this are not hard to find: they are available in profusion, are easily reduced without affecting other functional groups, and a variety of products can be produced, depending upon the exact nature of the experimental conditions. Studies on the electrochemical reduction of nitro compounds have produced important insights into the mechanistic pathways available to organic compounds generally. The electrochemical reduction of a given nitro compound may take a considerably different course when variables such as the pH, electrolysis potential or nature of the solvent are varied. This has made the study of their reduction mechanisms a popular and challenging research topic. Nitroso compounds are much less common than nitro compounds and have been studied far less. Nevertheless, their status as putative intermediates in the
837
838 |
Albert J. Fry |
electrochemical reductions of nitro compounds has led to a number of studies of their behavior.
II.GENERAL CONSIDERATIONS
A.Mechanism of Electrochemical Reduction
It will be helpful at this point to review a few well-known features of the electrochemical behavior of nitro and nitroso compounds. The reader is referred to a previous review in this series for more detail on this point1. The primary fact of which one must be aware of is that the electrochemistry of nitro compounds is exclusively cathodic: the high oxidation level of nitrogen in the nitro group means that while they are easily reduced, they generally cannot be oxidized. As a matter of fact, nitrobenzene and nitromethane have been used as solvents for electrochemical oxidations because of their stability under anodic conditions2. Nitroso compounds are readily both oxidized and reduced, although the literature on these substances is much more sparse.
1. Dependence of proton activity of medium
Aryl nitro compounds are by far the most common such substances, and nitrobenzene
(1) is the best known of these. Nitrobenzene exhibits a single four-electron voltammetric wave at pH 5 or above; a second two-electron wave is observed at more negative potentials at pH 4 or lower. The products of the electrochemical reduction of nitrobenzene in aqueous organic media were established a century ago in the classic work of Haber3. Reduction at room temperature in weakly acidic media (roughly pH 5 to 7) consumes four electrons per mole of 1 and affords phenylhydroxylamine (2); reduction under more powerfully reducing conditions (pH 4 and more negative potentials) affords aniline by reductive cleavage of the N O bond of 2. If the reaction is carried out under more vigorous conditions (stronger acid, higher temperatures), the product is p-aminophenol, formed by acid-catalyzed rearrangement of 2 (the so-called Wallach rearrangement). Zuman has summarized the dependence of the ultimate fate of the arylhydroxylamine (that is, whether it is isolated or undergoes further transformation) on experimental conditions4. In contrast to these multi-electron processes in protic media, 1 exhibits a one-electron wave followed by a second three-electron wave at more negative potentials. Controlled-potential electrolysis at relatively positive potentials affords a very stable radical anion in media of low proton availability (aprotic solvents or even aqueous alkali). Reduction in liquid ammonia as solvent affords not only the radical anion but also the corresponding nitrobenzene dianion, at more negative potentials5. Similar behavior is observed in highly purified dimethylformamide6. Preparative scale electrolysis in alkaline media usually affords azoxy compounds (3), although the corresponding azo compounds (4) and hydrazo compounds
(5) have been isolated from some electrolyses. The reasons for the diversity of products under alkaline conditions is still not fully clear. Azoxy compounds are reduced to azo compounds relatively easily, hence it would appear unlikely that 3 could ever be isolated from such electrolyses, but it appears that part of the reason is the fact that azoxy compounds often precipitate from solution, protecting them against further reduction7. In general these substances are produced by consecutive electrochemical reduction of 3 to 4 and finally to 5.
|
|
O− |
|
|
|
|
|||
C6 H5NO2 |
C6 H5NHOH |
|
|
|
|
|
|
|
|
C6 H5N |
|
NC6 H5 |
C6 H5N |
|
NC6 H5 |
C6 H5NHNHC6 H5 |
|||
|
|
||||||||
|
|
||||||||
(1) |
(2) |
(3) |
(4) |
|
(5) |
||||
18. The electrochemistry of nitro, nitroso, and related compounds |
839 |
Aliphatic nitro compounds exhibit rather different behavior from nitroaromatic compounds. Secondary and primary nitro compounds tend to produce oximes because the intermediate nitroso compound quickly tautomerizes to the oxime (equation 1). Under aprotic conditions the radical anions of primary and secondary nitro compounds are relatively stable; those derived from tertiary nitro compounds, on the other hand, eject nitrite ion relatively readily (equation 2)8.
|
2e |
|
|
|
|
|
|
R1R2CHNO2 |
! |
R1R2CHN |
D |
O |
! |
R1R2C NOH |
(1) |
|
HC |
|
|
D |
|
||
|
e |
|
|
|
|
|
|
R1R2R3CNO2 |
! R1R2R3CNO2 ž ! R1R2R3Cž C NO2 |
(2) |
|||||
Nitrogen in a nitro group is in the highest oxidation state which the element can exhibit while still bound to carbon. Under powerfully reducing conditions the nitro group can be reduced to an amino group, in which nitrogen exhibits its lowest oxidation state. A number of species of intermediate oxidation level are possible between these two extremes. The electrochemical reduction of nitro compounds does in fact give rise to a number of such intermediates. Some of these can be directly identified, while the existence of others can sometimes only be inferred. Furthermore, a variety of paths may interconnect the various intermediates, starting materials and products of the electrochemical reduction of a given nitro compound, depending on the particular experimental conditions being employed. Different nitro compounds may react by different paths under identical experimental conditions, and a given compound may give rise to the same product by several different paths under different experimental conditions. Consider the electrochemical reduction of nitrobenzene (1) to phenylhydroxylamine (2) in a weakly acidic protic medium. This process involves overall uptake of four electrons by 1. As we shall see, this can occur by a variety of mechanisms, of which only one possibility is shown in Scheme 1. This scheme
C6H5NO2 C e |
! C6H5NO2 ž |
(1) |
(6) |
6 C HC |
! C6H5NO2Hž |
|
(7) |
7 C e |
! C6H5N(OH)O |
|
(8) |
8 C HC |
! C6H5N(OH)2 C OH |
|
(9) |
9 ! C6H5NDO C H2O |
|
|
(10) |
10 C e |
! C6H5NO ž |
|
(11) |
11 C HC |
! C6H5NOHž |
|
(12) |
12 C e |
! [C6H5NOH] |
|
(13) |
13 C HC |
! C6H5NHOH |
|
SCHEME 1 |

840 |
Albert J. Fry |
differs in one significant detail from the mechanism of reduction of 1 presented in the previous1 review in this series: it is now known that cleavage of the N O bond involves dehydration of an intermediate N,N-dihydroxy compound (9). Previously it had been suggested that nitrosobenzene (10) was formed by loss of hydroxide ion from intermediate 7. In essence, Scheme 1 describes the gradual decrease in the oxidation level of nitrogen as electrons are successively added to 1. Four electrons are added overall, but since this would build up an unacceptably high charge on 1 if no other change were to take place, the organic species responds by addition of protons at each stage to maintain its structure near neutrality. Scheme 1 works well to rationalize the known conversion of 1 to 2 in weak acid. However, it greatly misrepresents the complexity of the reactions taking place. For example, nitrosobenzene (10) is easier to reduce than 1, hence it does not build up in solution but rather is reduced immediately upon formation. But, if nitrosobenzene is easier to reduce than nitrobenzene, this means that the nitrobenzene radical anion (6) is itself thermodynamically capable of reducing 10 to its radical anion 11. One therefore really ought to add to Scheme 1 another line representing electron transfer from 6 to 10 to produce 11 and regenerate 1. Thus, there are two ways in which the reduction of 10 to 11 can take place, depending on whether electron transfer to 10 takes place from the electrode or from 6, respectively. Because the reactant and the various intermediates are produced and react in a narrow zone (the reaction layer) close to the electrode surface, homogeneous solution electron exchange between 6 and 10 certainly does take place; in fact, it is probably the primary path for formation of 119. Likewise, we should also note that 6 and 11 may undergo homogeneous solution electron exchange with other intermediates in the redox chain10. One must also appreciate that in general the mechanism and even the products of reduction of any given nitro compound will be sensitive to reaction conditions, in particular pH and electrolysis potential. Often it is not readily apparent what the actual electroactive species is in a particular conversion. [An electroactive species is a chemical entity undergoing electron exchange from or to the electrode.] For example, one might legitimately inquire whether the actual electroactive material in the reduction of 10 is 10 itself or whether it is its conjugate acid, which being positively charged ought to be considerably easier to reduce than 10. This question is of particular mechanistic relevance for understanding electrolyses carried out in relatively strongly acidic solution, especially when one appreciates that a component of the medium, e.g. the protonated form of a compound, may be the electroactive substance even when it is present at very low equilibrium concentration11. It is clear that working out the mechanism of the electrochemical reduction of nitro compounds represents a very challenging problem indeed. There has been a great deal of previous work in this area, largely summarized in previous reviews1,7b,12.
The electrochemical reduction of nitrosobenzene (10) to phenylhydroxylamine (2) and nitrobenzene (1) to the N,N-dihydroxy compound (9) can be used to illustrate another point. Complex organic electrochemical processes typically involve a series of individual reactions each of which is one of two types: (a) so-called ‘E’, or electron transfer steps (either homogeneous to or from another component of the medium depending on whether one is discussing oxidations or reductions, respectively or heterogeneous to or from the electrode) and (b) ‘C’ steps, i.e. chemical conversions (all reactions other than electron transfer). The conversion of 10 to 2 formally involves addition of the elements of hydrogen or, more specifically, two electrons and two protons, to 10. A major aim of mechanistic studies on organic electrode processes is the determination of the particular sequence of electron transfers and chemical steps involved in the overall reaction. For the conversion of 1 to 9 or 10 to 2, all combinations of two E steps and two C steps must a priori be considered as possible13. Scheme 1 shows one possible way in which each of these conversions might take place, i.e. by alternate addition of electrons and protons to either 1 or 10. In the commonly used terminology of mechanistic electrochemistry, these
18. The electrochemistry of nitro, nitroso, and related compounds |
841 |
are so-called ‘ECEC’ processes. The term ECEC is therefore a shorthand method for describing the sequence in which the electron transfer and protonation steps take place. If one includes all possible permutations of two proton and two electron transfers, there are six distinct mechanistic possibilities for the conversion of 1 to 9 and 10 to 2 (4!/2!2!), or twelve if one recognizes that the second electron transfer could be either homogeneous or heterogeneous. Some of these sequences are highly improbable on chemical grounds. We may expect, for example, that the mechanism of reduction of 1 and 10 in acid will probably not be EECC, because the species produced by the first electron transfer are likely to be strong bases and would surely react with a proton before the second electron transfer could take place. Likewise, it is unlikely that conversion of 1 to 9 (or 10 to 2) proceeds via a CCEE mechanism; this would imply that the electroactive species is doubly protonated nitrobenzene, whereas it is known that 1 and 10 are not strong bases. Even if such chemically unreasonable steps were to be omitted, it is clear that the number of discrete paths by which 1 might in principle be converted into 2 is very large. It would be very cumbersome to present all of the likely paths in a format similar to that of Scheme 1, i.e. as a series of chemical reactions written in text form. It turns out to be much more convenient to represent the various possible mechanistic paths in graphical form. This is done in the following way. Consider an electrochemical reaction involving the conversion of a substance A to a product B and requiring one electron transfer and one chemical step. There are two possible mechanisms (EC or CE) for this process, depending on the order in which the two steps occur. We can represent this as a so-called ‘square’ mechanism, represented as in Scheme 2. The EC path is represented by the top and right-hand equations while the CE process is represented by the left-hand and bottom reactions.
ECprocess
A |
e− |
A− |
|||||
|
|
|
H+ |
||||
|
|
H+ |
|
|
|||
|
|
|
|
||||
|
|
|
e− |
|
|
|
|
A H+ |
B |
||||||
|
|||||||
|
|||||||
CE process
SCHEME 2. Simplest (four-component) square mechanism
This type of representation is readily extended to cover more complex cases. A oneelectron, two-proton reduction can be described by a so-called ‘ladder’ scheme14, whereas a two-electron, one-proton process can be described by a ‘fence’ scheme15. Reactions involving uptake of the elements of H2 are relatively common in organic electrochemistry; in fact, we have already encountered the conversion of 1 to 9 and 10 to 2, which are examples of this type of process (see Scheme 1). Such processes may be represented by a nine-component square (Scheme 3). A number of features are common to schemes such as Schemes 2 and 3. All species in a given vertical column are in the same oxidation state but differ in their degree of protonation. Conversely, all species in a given horizontal row correspond to the same degree of protonation but are in different oxidation states. Each path which may be traced between any one species on the diagram and any other corresponds to a distinct electrochemical mechanism. The ECEC mechanism (A ! A ! AH ! AH ! AH2) which was mentioned previously as a mechanistic path from 1 to 9 and 10 to 2 can be seen from Scheme 3 to be only one of a number of paths by which this conversion might take place.

842 |
|
|
|
|
|
Albert J. Fry |
|
|
||||||
A |
|
|
e− |
|
|
A− |
|
e− |
|
A−2 |
||||
|
|
|
|
|
|
|
|
|
||||||
|
|
|
H+ |
|
|
|
|
H+ |
|
|
|
H+ |
||
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
e− |
|
|
|
|
|
e− |
|
|
|
AH+ |
|
|
|
AH |
|
AH− |
||||||||
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
||||||||
|
|
|
H+ |
|
|
|
|
H+ |
|
|
|
H+ |
||
|
|
|
2 + |
e− |
|
|
|
|
+ |
e− |
|
|
||
AH |
|
AH |
|
AH |
||||||||||
|
|
|
|
|
||||||||||
2 |
|
|
2 |
|
|
2 |
||||||||
SCHEME 3. Nine-component square mechanism
As mentioned earlier, reduction of nitro compounds to hydroxylamines is now known to involve a sequence involving initial two-electron, two-proton conversion to an N,N- dihydroxy compound (9), dehydration of 9 to the corresponding nitroso compound (10) and finally a second two-electron, two-proton reduction of 10. We may now recognize that two nine-component square schemes, separated by the intervening dehydration of 9 to 10, are required to represent all possible mechanistic paths for this process in acidic media. In point of fact, however, the doubly protonated nitro and nitroso compounds, which correspond to the lower left-hand corner of Scheme 3, are improbable intermediates in these reactions, because 1 and 10 are weak bases and their doubly protonated forms will therefore be present in vanishingly small amounts even in strong acid. Likewise, the nitrobenzene and nitrobenzene dianions (corresponding to the top right-hand corners of Scheme 3) are equally improbable intermediates because they would be formed via intermediate monoanions, which would be very short-lived in acidic solution. For this reason, the top right and bottom left corners of Scheme 3 are sometimes omitted when describing the reduction of nitro compounds in acid, leaving a seven-component diagram consisting of two four-component square mechanisms sharing a single corner (Scheme 4). The common intermediate between the two four-component squares in Scheme 4 is the RN(OH)OÐradical (7).
C H NO |
|
e− |
|
C |
H NO |
− |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|||||||||
6 |
5 |
2 |
|
|
6 |
5 |
2 |
|
|
|
|
|
||||
|
|
|
H+ |
|
|
|
|
|
|
H+ |
|
|
|
|
|
|
|
|
|
|
|
e− |
|
|
|
|
|
|
e− |
||||
|
|
|
|
|
|
|
|
|
|
|
||||||
C6 H5NO2 H+ |
|
|
|
C6 H5N(OH)O |
|
|
C6 H5N(OH)O− |
|||||||||
|
|
|
|
|
||||||||||||
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H+ |
|
|
|
|
|
H+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C6 H5N(OH)OH+ |
e |
− |
|||||||
|
|
|
|
|
|
|
|
|
C6 H5N(OH)2 |
|||||||
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
||||||||
SCHEME 4
One of the advantages of electrochemical methods over more conventional chemical methods is the fact that the actual electron transfer process can be carried out at an electrode with a far greater degree of control than with a solution reactant. By careful application of the appropriate electrochemical techniques, it is possible to define the sequence of chemical and electron transfer steps in a given electrochemical process with

18. The electrochemistry of nitro, nitroso, and related compounds |
843 |
a high degree of specificity. Laviron has shown by such methods that the rate-determining step in the formation of the N,N-dihydroxy compound (9) is conversion of the radical ArN(OH)OÐ to 9. Using standard voltammetric techniques, he was able to conclude that the global mechanism for the reduction of ArNO2 to ArN(OH)2 changes from ECCE at pH D 0 to ECEC at pH D 5 (C is a protonation step) with the change in mechanism taking place at about pH D 316. With 4-nitrobenzophenone the rate of dehydration of the N,N-dihydroxy compound controls the rate of the first reduction step in strongly acidic media H0 D 2 17. The sequence of steps involved changes through CECE, ECCE and finally ECEC as the acidity of the medium increases from H0 D 5 to pH 10, and is EE at pH > 10. Dehydration of 9 is sometimes slow enough that the nitroso compound can be observed as a transient intermediate during electrolysis, for example at low temperature18 or with certain special structural types such as p-dinitrobenzene18 and the nitropyridines19.
Information obtained by voltammetric studies and preparative scale electrolyses can often be used to understand the course of reactions carried out with chemical reductants or oxidants. Nitro compounds provide an excellent illustration of this point. Organic chemists have known for many years that aryl nitro compounds can be reduced to a variety of compounds, depending on experimental conditions. For example, reduction to the corresponding aniline is often carried out using metallic tin or iron (strong reducing agents) and hydrochloric acid; this is consistent with the voltammetric data, which indicate that conversion of the hydroxylamine to the amine requires a relatively negative potential and a rather strongly acidic medium. Similarly, reduction of nitrobenzene (1) to azobenzene
(4) is typically carried out using a strong reductant, for example metallic zinc, in alkaline medium20; we have seen that dimeric derivatives are formed only under basic conditions and that the initial dimer is normally the azoxy compound (3), which is reduced further to the azo compound. Finally, reduction of the nitro compound under basic conditions with a mild reductant affords the azoxy compound, which can likewise be obtained by electrochemical reduction in base under mild conditions.
2. Other medium effects
From the discussions up to this point it should be clear that reduction of nitrobenzene in acidic media affords phenylhydroxylamine, whereas reduction in basic media affords azoxy compounds and/or their secondary electrolysis products, azo or hydrazo compounds. Ohkubo recently made the very interesting observation that azoxybenzene is the major product (together with some azobenzene and a trace of hydrazobenzene) when the solvent for electrochemical reduction of nitrobenzene (acetonitrile) is saturated with carbon dioxide21. The authors suggested that carbon dioxide emulates the effect of a proton in this reaction. This cannot be the entire answer, since addition of phenol to the medium instead of carbon dioxide results in formation of phenylhydroxylamine, not azoxybenzene22. Carbon dioxide probably acylates the electrochemically-generated nitrobenzene radical anion, paving the way for N O bond breakage by loss of carbonate ion and providing an alternate route to azoxybenzene to that shown in Scheme 1. A possible mechanism is presented in Scheme 5.
Thus far the solvent systems we have discussed are typical protic organic media, such as, for example, water ethanol mixtures containing an added supporting electrolyte. These solvents are presumably quite homogeneous on a microscopic level. However, a number of solvents have been developed in recent years which are heterogeneous on a microscopic scale. Micellar media are one example of such solvents. The electrochemical reduction of nitrobenzene in aqueous solutions containing polyoxyethylene lauryl ether, a substance known to produce neutral micelles, produces azobenzene (4) even at pH somewhat less than 723. This is apparently the first case of formation of a dimeric product from electrolysis of nitrobenzene (1) in acidic media. Another striking example of this phenomenon

844 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Albert J. Fry |
|
|
|
|
|||||||||||||
|
|
|
|
|
|
e− |
|
|
|
|
|
O− |
CO2 |
OCO2 |
− |
||||||||||||||||
|
|
|
|
|
|
C6 H5N + |
|
|
|
|
|||||||||||||||||||||
C6 H5NO2 |
|
|
|
|
|
|
|
|
|
|
|
C6 H5N + |
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O− |
|
|
|
|
|
|
|
|
|
|
O− |
|
|||
|
e |
|
|
|
|
|
|
OCO − |
− CO3 −2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
− |
C6 H5N |
2 |
|
|
|
|
C6 H5N |
|
|
|
|
|
O |
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
O− |
|
|
|
|
|
|
|
|
|
|
|
O− |
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
e− |
|
|
|
|
|
|
O− |
|
dimerization |
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
C6 H5N |
|
|
|
|
|
|
|
|
|
C6 H5N |
|
|
|
NC6 H5 |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
O− |
|
|
|
|
|
|
|
|
|
|
|
|
|
O− |
|
O− |
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
CO2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
− CO3 |
−2 |
|
|
|
|
|
|
|||||||||
|
|
C |
H N |
|
|
|
|
NC H |
|
|
C H N |
|
|
|
NC H |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
|
|
|
6 |
5 |
|
|
|
|
|
|
|
6 |
|
5 |
|
|
|
6 5 + |
|
6 |
5 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OCO |
− |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
SCHEME 5
was recently observed during electrochemical reduction of 1 in a so-called ‘microemulsion’ consisting of 34% didodecylammonium bromide (DDAB), 51% hexane and 15% 5M aqueous HCl24. Constant-current electrochemical reduction of 1 in this solvent affords a mixture of azobenzene and azoxybenzene! [Recall that these products are usually found only in electrolyses of nitrobenzene in alkaline meda.] Although microemulsions are thermodynamically stable and homogeneous on a macroscopic scale (for example, they do not scatter light), they are undoubtedly quite heterogeneous at the molecular level25. Nitrobenzene is presumably concentrated in the hydrocarbon phase in both of these media and therefore its local concentration is undoubtedly higher than its nominal concentration. As was also recognized by Blount23, this would favor dimerization, a bimolecular process.
As noted at the outset, electrochemistry normally occurs in a thin layer of solution near the electrode surface. Electrochemical reactions frequently occur via adsorbed reactants and/or intermediates. Whether adsorption effects are observed in a given situation depends not only on the structure of the electroactive substance but also on the nature of the solvent and, very importantly, the composition of the electrode. Adsorption effects fall into a variety of categories: reduction of a substance may become either easier or harder, some transformations may be totally inhibited and chemical reactions in adsorbed films may proceed at rates different than in homogeneous solution. For example, deposition of small amounts of palladium on a gold surface results in an electrode in which the 4- electron wave of some nitro compounds is shifted to more negative potentials and aniline formation is totally inhibited, but 3-nitro-1,2,4-triazole is easier to reduce than at pure gold, and the overall rate of reduction is controlled by the rate of dehydration of the ArN(OH)2 intermediate26. Under similar conditions 2- and 4-nitroimidazole are reduced by parallel pathways: (a) electron transfer from the electrode to afford the ArN(OH)2 intermediate and (b) electrocatalytically by adsorbed hydrogen (see below)27.
Reductions at noble metal electrodes in acidic protic media often form adsorbed hydrogen, which is the actual reductant. For example, reduction of nitrobenzene at a Pd/C electrode in acetic acid methanol mixtures affords aniline via adsorbed hydrogen28. This reaction is more closely related to catalytic hydrogenation of nitro groups than to the
18. The electrochemistry of nitro, nitroso, and related compounds |
845 |
electrochemical process. The same might be said of electrochemical reductions of nitro compounds using Devarda copper29, Raney nickel30,31 or Ti/TiO232 electrodes. Pintauro found that nitrobenzene could be reduced to aniline at Raney nickel when sodium tosylate is the supporting electrolyte, but that reduction went all the way to cyclohexylamine (!) when the supporting electrolyte is tetraethylammonium tosylate31.
III. SUBSTITUTED NITROAROMATICS
Laviron has studied an especially interesting class of nitro compounds containing a second basic site, e.g. 4-nitropyridine (14)33. Even two-dimensional representations such as those encountered earlier (Schemes 2 4) are inadequate to represent this mechanistically very complex situation. Laviron showed, however, that the electrochemical conversion of 14 to the corresponding ArN(OH)2 species can be satisfactorily explained in terms of a modified so-called ‘bi-cubic’ diagram (Figure 1). Note that the each of the front and rear planes of the bi-cubic model consists of a seven-component reaction diagram analogous to that of
+HNRNO2 |
|
|
|
+HNRNO2. |
|
|
|
|
|
|
||
NRNO2 |
|
NRNO2. |
|
Lower plane |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+HNRNO |
H+ |
|
|
+HNRNO |
H. |
|
|
+HNRNO H. |
|||
|
2 |
|
|
2 |
|
|
|
|
|
2 |
||
NRNO2H+ |
NRNO |
|
|
2H. |
NRNO |
|
2H. |
|
|
|||
|
|
|
|
|||||||||
|
|
|
|
+HNRN(OH)2+ |
|
|
|
+ |
HNRN(OH)2 |
|||
Upper plane |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NRN(OH)2+ |
NRN(OH)2 |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
+HNRN(OH)(OH2+) |
|
|
|
|
|
|
|
|
NRN(OH)(OH2+) |
|||||
|
|
−H2O k |
|
|
|
|
|
|
||||
|
|
R′NO |
|
|
|
|
|
|
||||
|
Bi-cubic scheme |
|
|
2e, 2H+ |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||||
|
(R′ = +HN or N) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R′NHOH
FIGURE 1. Bicubic mechanism for reduction of nitropyridines. Reproduced by permission of Elsevier Science SA from Reference 33
846 |
Albert J. Fry |
Scheme 4. The compounds and intermediates on the ‘rear’ plane of the bicubic system (farthest from the reader) are protonated on the pyridine nitrogen atom; those on the ‘front’ plane (nearest the reader) are not. Laviron’s work has shown that the reduction of 14 and its corresponding N-oxide34, and indeed probably most aryl nitro compounds, proceeds by an ECEC sequence leading to the neutral N,N-dihydroxy [ArN(OH)2] intermediate at all proton concentrations from H0 D 6 to pH 9.6. This substance then loses water to form the nitroso compound, which then undergoes a second sequence leading to the arylhydroxylamine.
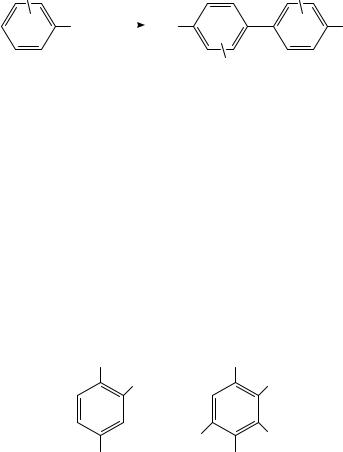
Pentahalonitrobenzenes (15) undergo electrochemical coupling to the corresponding octahalobiphenyls (16, equation 3)35. There is an interesting mechanistic dichotomy between the fluorine and chlorine compounds (15a and 15b, respectively). The radical anion of 15a couples, then the resulting dimeric dianion ejects two fluoride ions to afford 16; in contrast, the radical anion of 15b ejects chloride ion to afford a neutral radical, which then dimerizes to 16.
X5 |
|
|
|
X4 |
|
|
2 e− |
|
|
|
NO2 |
|
O2 N |
NO2 |
|
− 2 X− |
|||
|
|
|
|
(3) |
|
|
|
|
X4 |
(15a) |
X = F |
(16) |
||
(15b) |
X = Cl |
|
||
It was noted at the outset (Section II.A.1 that reduction of nitro compounds in basic media generally affords dimeric products (azoxy, azo or hydrazo compounds). It has been found, however, that reduction in 1N NaOH of nitroarenes bearing electron-supplying groups, especially hydroxy and alkoxy groups, affords amines in good yields36. This is presumably because the intermediate hydroxylamine dehydrates readily to a quinoid substance, which then undergoes facile reduction to the amine (equation 4). Similar conversion to the amine was observed with a naphthalenic nitrosophenol (equation 5)37. p-Nitrodiphenylamine is reduced all the way to the amine via dehydration of the intermediate hydroxylamine; however, reduction of the corresponding N-acylated compound stops at the hydroxylamine, which undergoes dehydration much less readily36b. In a related vein, it was reported that whereas 2-methyl-5-nitroaniline (17) exhibits a four-electron wave followed by a two-electron voltammetric wave in acidic medium, the closely related substance 4,6-di-t-butyl-2-methyl-3-nitroaniline (18) exhibits a single six-electron wave at the same pH. This suggests that the intermediate hydroxylamine from 18 is reduced to the corresponding amine faster than that from 17. The authors ascribed this difference to the larger number of alkyl groups in 18 causing the hydroxylamine formed from it to be more basic than that from 1738. It seems more likely that the increased basicity arises because
CH3 |
Bu-t |
NH2 |
NH2 |
t-Bu |
CH3 |
NO2 |
NO2 |
(17) |
(18) |
