
Environmental Biotechnology - Theory and Application - G. M. Evans & J. C. Furlong
.pdf10
Integrated Environmental
Biotechnology
The essence of environmental biotechnology as an applied science, as we have set out to demonstrate in the preceding chapters of this book, is the harnessing of pre-existing organisms and natural cycles to bring about a desired goal. Sometimes this is achieved by relatively unsophisticated means. At others it requires rather more in the way of engineering, adaptation or modification, in one form or another, to fit nature’s original to the intended purpose. Thus, though the exact form of any given iteration may differ, the underlying paradigm remains the same. Applying what is effectively a naturalistic model leads to some inevitable conclusions with far-reaching implications for the future of this particular discipline.
The fundamental necessity of mutual interactions in nature is readily accepted and understood. Hence, the natural cycles obligatorily dovetail together at both the gross and the microscopic levels, with interplay existing between the organism and its environment as well as between the various central metabolic pathways. Since such integration exists already between bioprocesses, and these are the very stuff upon which environmental biotechnology is based, the potential for integrated applications is clear.
At its simplest, this involves the sequential use of individual technologies to provide a solution in a linked chain of successive steps, often termed a ‘treatment train’. The other extreme is the wider amalgamation of larger fundamental problems and their resolutions into a single cohesive whole. This book began by looking at the key intervention areas for environmental biotechnology and defined the three legs of that particular tripod as pollution, waste and manufacturing. This theme has been further developed, to examine how old pollution can be cleaned up and how the rational treatment of solid wastes and effluent can contribute to the reduction of new pollution. So-called ‘clean’ technologies represent the logical end-point of this discussion, when the production processes themselves assist in the reduction of waste and the minimisation of pollution, in the ultimate integrated system.
All industrialised countries face the same three problems in attempting to marry economic growth with environmental responsibility, namely the need to marshal material resources, deal rationally with their waste and the requirement for adequate and affordable energy. This dichotomy of desire between compromising neither
236 Environmental Biotechnology
commercial success nor environmental stewardship is particularly important for the long-term future of the economy. Over the years, a certain brand of extremist environmentalist thought has sought to demonise industry and commerce, decrying them and casting them in the role of enemy. This is scarcely helpful, for two reasons. Firstly, if any particular industry is actively damaging the environment, it is hardly likely to react constructively to criticism from its avowed detractors. Secondly, and perhaps much more importantly, industry in its widest sense is what has defined humanity from the outset. It accounts for what our Neolithic ancestors did, trading skins and flint axes across Europe; it is absurd to suggest that our collective future will be different. The way ahead, then, is to accept this and chart a course which, if it cannot do the most good in absolute terms, must settle for doing the least harm. In much the same way as some have vilified industry, there are those who have held the idea of a self-sustaining civilisation up to ridicule, arguing that ultimately this would have us living in mud huts, devoid of all the benefits of science and technology. The one view is as facile as the other.
The issue of sustainability has gained ever greater significance over recent years, and this seems set to continue in the future. In 1987, under the aegis of the World Commission on Environment and Development, the Bruntland Commission coined a definition of sustainable development. Their concept of an approach which ‘meets the needs of the present without compromising the ability of future generations to meet their own needs’ has received widespread international acceptance. The main aims have been further developed into social progress to address the requirements of all, effective environmental stewardship, the maintenance of high and stable economic growth and levels of employment, and the utilisation of natural resources in a prudent fashion (DETR 1999). These goals also tend to offer strong commercial benefits and as a result, businesses have not been slow to see their potential. In a survey undertaken by the management consultancy, Arthur D. Little, of some 500 environmental, health and safety and other business executives in North America and Europe, 95% believed sustainable development was ‘important’. Around 80% said it had significant real business value, while 70% of the Europeans and more than 55% in the USA reported an active sustainable development approach to strategy and operations within their organisations, for reasons of perceived business advantage. In this context, increased efficiency, competitive streamlining, better public relations, work-force awareness and rising customer expectations were all cited, while the impact of technological innovation was universally recognised.
In many respects, the move towards integration is inevitable. We cannot unscrew one leg of our tripod without unbalancing the whole structure. Sustainable development inherently demands a cogent view of resource management, and this implicitly covers materials, waste and energy. It becomes impossible to consider them in isolation. If waste becomes viewed as raw-material-in-waiting, one bridge is clear. Between waste and energy, however, the current link is incineration and, although this route will always be relevant for some unwanted materials, the
Integrated Environmental Biotechnology 237
situation is less than ideal. For one thing, burning denies the bridge discussed above, by allowing little or no opportunity for reclamation. If we extend this to larger environmental issues, like reducing CO2 production and the usage of fossil fuels, biomass, and hence environmental biotechnology, comes to occupy a pivotal position in the sustainability debate.
Bioenergy
The concept of obtaining energy from biomass material was mentioned earlier, in respect of the biological waste treatment methods involving anaerobic digestion and fermentation, and represents nothing particularly novel in itself. Methane and ethanol have been long established as fuels in many parts of the world, their production and utilisation being well documented. Both of these may be described as derived fuels, biochemically obtained from the original biomass. However, to many people around the globe, the most familiar forms of biofuel are far more directly utilised, commonly via direct combustion and, increasingly, pyrolysis. Around half the world’s population relies on wood or some other form of biomass to meet daily domestic needs, chiefly cooking. Estimates put the average daily consumption of such fuels at between 0.5 – 1.0 kg per person (Twidell and Weir 1994a). This equates to around 150 W which is an apparently high figure, but one largely explained by the typical 5% thermal efficiency of the open-fire method most commonly encountered.
The energy demands of the developed world are well known to be enormous. In the USA alone, the requirement for electricity has grown by 2.7% on average per year over the past 10 years (Perkowitz 2000). The Executive Order on Biobased Products and Bioenergy, August 1999, set out the goal of tripling US biomass use by 2010, which has been estimated to be worth around $15 billion of new income, while at the same time reducing carbon emissions by the equivalent of removing some 70 million cars from the road (Feinbaum 1999). The European Commission has also suggested that the EU as a whole should aim to double the current contribution made by renewable energy sources, taking it to 12%, also by 2010. Under this proposal, biomass energy was to provide an additional 90 million tonnes of oil equivalent (Mtoe) per year, raising its overall share to 137 Mtoe. Half of this would come from specifically farmed energy crops, while other biofuel forms would account for the rest.
The energy of all biofuels derives ultimately from the sun, when incident solar radiation is captured during photosynthesis, as discussed in Chapter 2. This process collects around 2 × 1021 joules of energy, or 7 × 1013 watts, each year, throughout the biosphere as a whole. During biomass combustion, as well as in various metabolic processes described elsewhere, organic carbon reacts with oxygen, releasing the energy once more, principally as heat. The residual matter itself feeds back into natural cycles for reuse. It has been calculated that a yearly total of some 2.5 × 1011 tonnes of dry matter circulates around the biosphere, in

238 Environmental Biotechnology
Figure 10.1 The biomass and bioenergy cycle
one form or another, of which around 1 × 1011 tonnes are carbon. (Twidell and Weir 1994b).
This relationship of energy and matter within the biospheric system, shown schematically in Figure 10.1, is of fundamental importance to understanding the whole question of biomass and biofuels. Before moving on to examine how integrated technologies themselves combine, it is worth remembering that the crux of this particular debate ultimately centres on issues of greenhouse gases and global warming. Increasingly the view of biomass as little more than a useful long-term carbon sink has been superseded by an understanding of the tremendous potential resource it represents as a renewable energy. Able to substitute for fossil fuels, bioenergy simply releases the carbon it took up during its own growth. Thus, only ‘modern’ carbon is returned, avoiding any unwanted additional atmospheric contributions of ancient carbon dioxide.
Derived Biofuels
Methane biogas
Biogas is a methane-rich gas resulting from the activities of anaerobic bacteria, responsible for the breakdown of complex organic molecules. It is combustible, with an energy value typically in the range of 21 – 28 MJ/m3. The general processes of anaerobic digestion and the biochemistry of methanogenesis have been discussed in earlier sections of this book, so they will not be restated here. As mentioned previously, the main route for methane production involves acetic acid/acetate and accounts for around 75% of gas produced. The remainder is made up via methanol or carbon dioxide and hydrogen, as shown in Figure 10.2.
At various times a number of models have been put forward to aid the prediction of biogas production, ranging from the simplistic to the sophisticated.

Integrated Environmental Biotechnology 239
|
Hydrogenotrophes |
Acetoclasts |
|
|
|
Figure 10.2 The methanisation of biowaste
Many of these have been based more on landfill gas (LFG) generation than truly representative anaerobic bioreactors, which does lead to some confusion at times. However, it is generally accepted that the linked, interdependent curves for cellulose decomposition and gas evolution can be broadly characterised as having five principal stages, outlined below.
•Stage I – Peak biowaste cellulose loadings; dissolved oxygen levels fall to zero; nitrogen, and carbon dioxide tend to atmospheric levels.
•Stage II – Carbon dioxide, hydrogen and free fatty acids levels peak; nitrogen levels fall to around 10%; cellulose begins to be broken down.
•Stage III – Carbon dioxide decreases and plateaus, to hold at around 40%; methane production commences and achieves steady state at around 60%; free fatty acids decrease to minimum levels; cellulose breakdown continues at a linear rate with respect to time; nitrogen levels fall to near zero.
•Stage IV – Carbon dioxide and methane continue in steady state at c. 40% and c. 60% respectively; cellulose component reduces steadily.
240 Environmental Biotechnology
•Stage V – Cellulose becomes fully decomposed, ultimately leading to zero methane and carbon dioxide production; oxygen and nitrogen revert to atmospheric levels.
Although beyond the scope of the present discussion to address fully, the position of hydrogen as a regulator of methane production warrants a brief mention. In the earlier examination of anaerobic digestion the obligate syntrophic relationship between the hydrogen-producing acetogenic bacteria and the hydrogen-utilising methanogens, was described. Essentially, higher fatty acids and alcohols are converted to acetate, which requires an active population of hydrogenotrophic methanogens to ensure a low hydrogen partial pressure, avoiding the preferential production of butyric, lactic, proprionic and other acids instead of the desired acetic. This has the potential to cause higher volatile fatty acids to accumulate beyond the system’s ability to self-buffer, leading to a lowering of the pH. In turn, as the increased acidity inhibits the methanogens themselves, methane production ceases and ultimately the process will collapse.
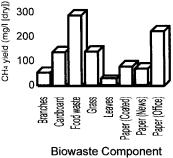
A number of different applications have developed the idea of anaerobic digestion for methane production, notably in the waste management, sewage treatment, agricultural and food processing industries. The process has also been successfully used at relatively small scale, commonly with animal manures as its feedstock. Figure 10.3 shows an illustrative chart of methane generation for many of the common biodegradable components of MSW.
Methane has an explosive range of 5 – 15% by volume and a density at 20 ◦C of 0.72 kg/m3; for hydrogen the same properties lie between 4 – 74% and 0.09 kg/m3 at 20 ◦C, respectively. At 20 ◦C, carbon dioxide has a density of 1.97 kg/m3. The calorific value of typical biogas, consisting of about 60% CH4, 40% CO2, lies between 5.5 – 6.5 kWh/m3 and it is this which makes its production attractive as a means of generating renewable energy. As was mentioned in the earlier section on anaerobic digestion, with a theoretical yield of 400 m3 of biogas per wet cellulosic tonne, the prospect of high energy returns simultaneous with waste
Figure 10.3 Methane generated from biowaste components
Integrated Environmental Biotechnology 241
treatment has clear appeal. However, as was also pointed out in the same earlier segment, it is not feasible to optimise conditions such that high levels of both waste reduction and gas generation are deliverable. More commonly, in practice only around a quarter of the potential biogas yield is actually achieved.
Using biogas
Although biogas from engineered AD processes share many similarities with landfill gas (LFG), it is important to remember that it is of quite distinct quality, being much cleaner and far less contaminated by traces of other gases. LFG may contain a bewildering array of ‘others’, dependent on the exact nature of the waste undergoing decomposition. The list includes the likes of 1,2-dichloroethene, alkylbenzene, butylcyclohexane, carbon disulphide, propylcyclohexan, methanethiol, decane, dichlorobenzene, undecane, ethylbenzene, dodecane, trimethylbenzene, tridecane, toluene, dimethyl disulphide, nonane and sulphur dioxide. Biogas, by contrast, is relatively pure by comparison, since the bulk of the inorganic matter and many potential pollutants are excluded from the bioreactor, either by source or mechanical separation, as part of the waste preparation process. This obviates the need for high temperature flaring, commonly used for LFG to destroy residual pollutant gases, since they are highly hostile to the fabric of any generation equipment intended to be used.
The main cause for concern in this respect is hydrogen sulphide (H2S), which is a metabolic byproduct of sulphur-reducing bacteria. Unsurprisingly, the amount present in the final gas produced depends largely on the relative abundance of sulphur-containing compounds in the original biowaste. H2S is acidic and this poses a major corrosion risk to gas handling and electrical generation equipment. Scrubbing hydrogen sulphide out of biogas is possible, but in practice it is more common to use a high-alkalinity lubricant oil which is changed often.
Biogas utilisation involves burning it, with some of the energy being transformed to electrical. There are three basic types of engine which are suitable generating motors for biogas uses, namely turbine, dual fuel and spark ignition. For each there are numbers of different manufacturers worldwide. While, clearly, it lies far outside of the scope of this book to discuss them, it is worth noting that for any given application, the type of engine used will normally be decided by a number of contextual issues. Hence, the quantity of the biogas produced, its purity, the intended life of the plant, relevant pollution controls and other similar site-specific considerations will need to be considered.
Generation processes are generally relatively inefficient thermodynamically, and often much of the available energy is effectively lost as heat. However, the nature of engineered AD processes are such that there is a ready built-in demand for thermal energy to elevate and maintain the digester temperature. This may account for between 20 – 50% of the total energy produced, dependent on system specifics, in a typical temperate facility, with the remainder being available for

242 Environmental Biotechnology
Figure 10.4 Energy from biogas utilisation
other uses. A representative energy flow for gas-engine generators is shown in Figure 10.4.
Ethanol fermentation
Fermentative processes have been described earlier, both in the general wider metabolic context and specifically in regard to their potential use in the treatment of biowaste. Fermentation produces a solution of ethanol in water, which can be further treated to produce fuel-grade ethanol by subsequent simple distillation, to 95% ethanol, or to the anhydrous form by azeotropic codistillation using a solvent.
The relative ease with which liquid fuels can be transported and handled, coupled with their straightforward delivery to, and inherent controllability of combustion in, engines makes them of considerable importance. Ethanol is a prime example in this respect, since it can be used either as a direct replacement for petrol, or as a co-constituent in a mix. Though at 24 G J/m3, it has a lower calorific value than petrol (39 G J/m3), in practice any performance discrepancy is largely offset by its better combustion properties.
There are thriving ethanol industries in many countries of the world, generally using specifically energy-farmed biomass in the form of primary crop plants, like corn in the USA and sugarcane in Brazil. In another example of the importance of local conditions, the production costs of ethanol and the market price realised by the final fuel depend on many factors external to the technology itself. Hence the indigenous economy, employment and transport costs, government policy, taxation instruments and fiscal incentives all contribute to the overall commercial viability of the operation.
Brazil, where ethanol/petrol mixing has been routine since the 1970s is an excellent example. Although the country’s use of ethanol partial substitution has a relatively long history, dating back to the 1930s, the real upsurge of acceptance of ‘gasohol’ lay in an unusual combination of events, partly driven by the energy crisis of the mid-1970s. Rising oil prices, which increased by over 25% in less
Integrated Environmental Biotechnology 243
than two years, came at the same time as a fall in sugar revenue following a slump in the world market. The Brazilian sugarcane industry, which had shortly before invested heavily in an extensive national programme of modernisation, faced collapse. Against this background, the production of fuel from the newly available biomass crop became a sound commercial move, simultaneously reducing the country’s outlay on purchased energy and buoying up one of its major industries.
The keynote of this chapter is the potential for integrating biotechnologies. In the preceding discussion of biogas, this involved the marrying together of the goals of biowaste treatment and energy production. In a similar vein, as was described in an earlier chapter, there have been various attempts, over the years, to produce ethanol from various forms of waste biomass, using naturally occurring microbes, isolated enzymes and genetically modified organisms (GMOs). The appeal to obtaining renewable energy from such a cheap and readily available source, is obvious.
In many respects, the situation which exists today with biowaste is very similar to that which surrounded Brazil’s sugarcane, principally in that there is an abundant supply of suitable material available. The earlier technological barriers to the fermentation of cellulose seem to have been successfully overcome. The future of ethanol-from-biowaste as an established widespread bioindustrial process will be decided, inevitably, on the long-term outcome of the first few commercial projects. It remains fairly likely, however, that the fledgling industry will depend, at least initially, on a sympathetic political agenda and a supportive financial context to succeed. While this application potentially provides a major contribution to addressing two of the largest environmental issues of our time; energy and waste, it is not the only avenue for integrated biotechnology in connection with ethanol production.
As has already been mentioned, specifically grown crops form the feedstock for most industrial fermentation processes. The distillation which the fermentate undergoes to derive the final fuel-grade alcohol gives rise to relatively large volumes of potentially polluting byproducts in the form of ‘stillage’. Typically high in BOD and COD, between six and 16 litres are produced for every litre of ethanol distilled out. A variety of end-use options have been examined, with varying degrees of success, but dealing with stillage has generally proved expensive. Recently developments in anaerobic treatments have begun to offer a better approach and though the research is still at an early stage, it looks as if this may ultimately result in the double benefit of significantly reduced cost and additional biomass to energy utilisation. The combination of these technologies is itself an interesting prospect, but it opens the door for further possibilities in the future. Of these, perhaps the most appealing would be a treatment train approach with biowaste fermentation for ethanol distillation, biogas production from the stillage and a final aerobic stabilisation phase; an integrated process on a single site. There is, then, clear scope for the use of sequential, complementary approaches in this manner to derive maximum energy value from waste biomass
