
- •Preface and Acknowledgments
- •Contents
- •Contributors
- •1: Embryology for Urologists
- •Introduction
- •Renal Development
- •Pronephros
- •Mesonephros
- •Metanephros
- •Development of the Collecting System
- •Critical Steps in Further Development
- •Anomalies of the Kidney
- •Renal Agenesis
- •Renal Aplasia
- •Renal Hypoplasia
- •Renal Ectopia
- •Renal Fusion
- •Ureteral Development
- •Anomalies of Origin
- •Anomalies of Number
- •Incomplete Ureteral Duplication
- •Complete Ureteral Duplication
- •Ureteral Ectopia
- •Embryology of Ectopia
- •Clinical Correlation
- •Location of Ectopic Ureteral Orifices – Male (in Descending Order According to Incidence)
- •Symptoms
- •Ureteroceles
- •Congenital Ureteral Obstruction
- •Pipestem Ureter
- •Megaureter-Megacystis Syndrome
- •Prune Belly Syndrome
- •Vascular Ureteral Obstructions
- •Division of the Urogenital Sinus
- •Bladder Development
- •Urachal Anomalies
- •Cloacal Duct Anomalies
- •Other Bladder Anomalies
- •Bladder Diverticula
- •Bladder Extrophy
- •Gonadal Development
- •Testicular Differentiation
- •Ovarian Differentiation
- •Gonadal Anomalies
- •Genital Duct System
- •Disorders of Testicular Function
- •Female Ductal Development
- •Prostatic Urethral Valves
- •Gonadal Duct Anomalies
- •External Genital Development
- •Male External Genital Development
- •Female External Genital Development
- •Anomalies of the External Genitalia
- •References
- •2: Gross and Laparoscopic Anatomy of the Upper Urinary Tract and Retroperitoneum
- •Overview
- •The Kidneys
- •The Renal Vasculature
- •The Renal Collecting System
- •The Ureters
- •Retroperitoneal Lymphatics
- •Retroperitoneal Nerves
- •The Adrenal Glands
- •References
- •3: Gross and Laparoscopic Anatomy of the Lower Urinary Tract and Pelvis
- •Introduction
- •Female Pelvis
- •Male Pelvis
- •Pelvic Floor
- •Urinary Bladder
- •Urethra
- •Male Urethra
- •Female Urethra
- •Sphincter Mechanisms
- •The Bladder Neck Component
- •The Urethral Wall Component
- •The External Urethral Sphincter
- •Summary
- •References
- •4: Anatomy of the Male Reproductive System
- •Testis and Scrotum
- •Spermatogenesis
- •Hormonal Regulation of Spermatogenesis
- •Genetic Regulation of Spermatogenesis
- •Epididymis and Ductus Deferens
- •Accessory Sex Glands
- •Prostate
- •Seminal Vesicles
- •Bulbourethral Glands
- •Penis
- •Erection and Ejaculation
- •References
- •5: Imaging of the Upper Tracts
- •Anatomy of the Upper Tracts and Introduction to Imaging Modalities
- •Introduction
- •Renal Upper Tract Basic Anatomy
- •Modalities Used for Imaging the Upper Tracts
- •Ultrasound
- •Radiation Issues
- •Contrast Issues
- •Renal and Upper Tract Tumors
- •Benign Renal Tumors
- •Transitional Cell Carcinoma
- •Renal Mass Biopsy
- •Renal Stone Disease
- •Ultrasound
- •Plain Radiographs and IVU
- •Renal Cystic Disease
- •Benign Renal Cysts
- •Hereditary Renal Cystic Disease
- •Complex Renal Cysts
- •Renal Trauma
- •References
- •Introduction
- •Pathophysiology
- •Susceptibility and Resistance
- •Epidemiological Breakpoints
- •Clinical Breakpoints
- •Pharmacodynamic Parameters
- •Pharmacokinetic Parameters
- •Fosfomycin
- •Nitrofurantoin
- •Pivmecillinam
- •b-Lactam-Antibiotics
- •Penicillins
- •Cephalosporins
- •Carbapenems
- •Aminoglycosides
- •Fluoroquinolones
- •Trimethoprim, Cotrimoxazole
- •Glycopeptides
- •Linezolid
- •Conclusion
- •References
- •7: An Overview of Renal Physiology
- •Introduction
- •Body Fluid Compartments
- •Regulation of Potassium Balance
- •Regulation of Acid–Base Balance
- •Diuretics
- •Suggested Reading
- •8: Ureteral Physiology and Pharmacology
- •Ureteral Anatomy
- •Modulation of Peristalsis
- •Ureteral Pharmacology
- •Conclusion
- •References
- •Introduction
- •Afferent Signaling Pathways
- •Efferent Signaling
- •Parasympathetic Nerves
- •Sympathetic Nerves
- •Vesico-Spinal-Vesical Micturition Reflex
- •Peripheral Targets
- •Afferent Signaling Mechanisms
- •Urothelium
- •Myocytes
- •Cholinergic Receptors
- •Muscarinic Receptors
- •Nicotinic Receptors
- •Adrenergic Receptors (ARs)
- •a-Adrenoceptors
- •b-Adrenoceptors
- •Transient Receptor Potential (TRP) Receptors
- •Phosphodiesterases (PDEs)
- •CNS Targets
- •Opioid Receptors
- •Serotonin (5-HT) Mechanisms
- •g-Amino Butyric Acid (GABA) Mechanisms
- •Gabapentin
- •Neurokinin and Neurokinin Receptors
- •Summary
- •References
- •10: Pharmacology of Sexual Function
- •Introduction
- •Sexual Desire/Arousal
- •Endocrinology
- •Steroids in the Male
- •Steroids in the Female
- •Neurohormones
- •Neurotransmitters
- •Dopamine
- •Serotonin
- •Pharmacological Strategies
- •CNS Drugs
- •Enzyme-inducing Antiepileptic Drugs
- •Erectile Function
- •Ejaculatory Function
- •Premature Ejaculation
- •Abnormal Ejaculation
- •Conclusions
- •References
- •Epidemiology
- •Calcium-Based Urolithiasis
- •Uric Acid Urolithiasis
- •Infectious Urolithiasis
- •Cystine-Based Urolithiasis
- •Aims
- •Who Deserves Metabolic Evaluation?
- •Metabolic Workup for Stone Producers
- •Medical History and Physical Examination
- •Stone Analysis
- •Serum Chemistry
- •Urine Evaluation
- •Urine Cultures
- •Urinalysis
- •Twenty-Four Hour Urine Collections
- •Radiologic Imaging
- •Medical Management
- •Conservative Management
- •Increased Fluid Intake
- •Citrus Juices
- •Dietary Restrictions
- •Restricted Oxalate Diet
- •Conservative Measures
- •Selective Medical Therapy
- •Absorptive Hypercalciuria
- •Thiazide
- •Orthophosphate
- •Renal Hypercalciuria
- •Primary Hyperparathyroidism
- •Hyperuricosuric Calcium Oxalate Nephrolithiasis
- •Enteric Hyperoxaluria
- •Hypocitraturic Calcium Oxalate Nephrolithiasis
- •Distal Renal Tubular Acidosis
- •Chronic Diarrheal States
- •Thiazide-Induced Hypocitraturia
- •Idiopathic Hypocitraturic Calcium Oxalate Nephrolithiasis
- •Hypomagnesiuric Calcium Nephrolithiasis
- •Gouty Diathesis
- •Cystinuria
- •Infection Lithiasis
- •Summary
- •References
- •12: Molecular Biology for Urologists
- •Introduction
- •Inherited Changes in Cancer Cells
- •VEGR and Cell Signaling
- •Targeting mTOR
- •Conclusion
- •References
- •13: Chemotherapeutic Agents for Urologic Oncology
- •Introduction
- •Bladder Cancer
- •Muscle Invasive Bladder Cancer
- •Metastatic Bladder Cancer
- •Conclusion
- •Prostate Cancer
- •Other Chemotherapeutic Drugs or Combinations for Treating HRPC
- •Conclusion
- •Renal Cell Carcinoma
- •Chemotherapy
- •Immunotherapy
- •Angiogenesis Inhibitor Drugs
- •Conclusion
- •Testicular Cancer
- •Stage I Seminoma
- •Stage I non-seminomatous Germ Cell Tumours (NSGCT)
- •Metastatic Germ Cell Tumours
- •Low-Volume Metastatic Disease (Stage II A/B)
- •Advanced Metastatic Disease
- •Salvage Chemotherapy for Relapsed or Refractory Disease
- •Conclusion
- •Penile Cancer
- •Side Effects of Chemotherapy
- •Conclusion
- •References
- •14: Tumor and Transplant Immunology
- •Antibodies
- •Cytotoxic and T-helper Cells
- •Immunosuppression
- •Induction Therapy
- •Maintenance Therapy
- •Rejection
- •Posttransplant Lymphoproliferative Disease
- •Summary
- •References
- •15: Pathophysiology of Renal Obstruction
- •Causes of Renal Obstruction
- •Effects on Prenatal Development
- •Prenatal Hydronephrosis
- •Spectrum of Renal Abnormalities
- •Renal Functional Changes
- •Renal Growth/Counterbalance
- •Vascular Changes
- •Inflammatory Mediators
- •Glomerular Development Changes
- •Mechanical Stretch of Renal Tubules
- •Unilateral Versus Bilateral
- •Limitations of Animal Models
- •Future Research
- •Issues in Patient Management
- •Diagnostic Imaging
- •Ultrasound
- •Intravenous Urography
- •Antegrade Urography and the Whitaker Test
- •Nuclear Renography
- •Computed Tomography
- •Magnetic Resonance Urography
- •Hypertension
- •Postobstructive Diuresis
- •References
- •Introduction
- •The Normal Lower Urinary Tract
- •Anatomy
- •Storage Function
- •Voiding Function
- •Neural Control
- •Symptoms
- •Flow Rate and Post-void Residual
- •Voiding Cystometry
- •Male
- •Female
- •Neurourology
- •Conclusions
- •References
- •17: Urologic Endocrinology
- •The Testis
- •Normal Androgen Metabolism
- •Epidemiological Aspects
- •Prostate
- •Brain
- •Muscle Mass and Adipose Tissue
- •Bones
- •Ematopoiesis
- •Metabolism
- •Cardiovascular System
- •Clinical Assessment
- •Biochemical Assessment
- •Treatment Modalities
- •Oral Preparations
- •Parenteral Preparations
- •Transdermal Preparations
- •Side Effects and Treatment Monitoring
- •Body Composition
- •Cognitive Decline
- •Bone Metabolism
- •The Kidneys
- •Endocrine Functions of the Kidney
- •Erythropoietin
- •Calcitriol
- •Renin
- •Paraneoplastic Syndromes
- •Hypercalcemia
- •Hypertension
- •Polycythemia
- •Other Endocrine Abnormalities
- •References
- •General Physiology
- •Prostate Innervation
- •Summary
- •References
- •Wound Healing
- •Inflammation
- •Proliferation
- •Remodeling
- •Principles of Plastic Surgery
- •Tissue Characteristics
- •Grafts
- •Flap
- •References
- •Lower Urinary Tract Symptoms
- •Storage Phase
- •Voiding Phase
- •Return to Storage Phase
- •Urodynamic Parameters
- •Urodynamic Techniques
- •Volume Voided Charts
- •Pad Testing
- •Typical Test Schedule
- •Uroflowmetry
- •Post Voiding Residual
- •Further Diagnostic Evaluation of Patients
- •Cystometry with or Without Video
- •Cystometry
- •Videocystometrography (Cystometry + Cystourethrography)
- •Cystometric Findings
- •Comment:
- •Measurements During the Storage Phase:
- •Measurements During the Voiding Phase:
- •Abnormal Function
- •Disorders of Sensation
- •Causes of Hypersensitive Bladder Sensation
- •Causes of Hyposensitive Bladder Sensation
- •Disorders of Detrusor Motor Function
- •Bladder Outflow Tract Dysfunction
- •Detrusor–Urethral Dyssynergia
- •Detrusor–Bladder Neck Dyssynergia
- •Detrusor–Sphincter Dyssynergia
- •Complex Urodynamic Investigation
- •Urethral Pressure Measurement
- •Technique
- •Neurophysiological Evaluation
- •Conclusion
- •References
- •Endoscopy
- •Cystourethroscopy
- •Ureteroscopy and Ureteropyeloscopy
- •Nephroscopy
- •Virtual Reality Simulators
- •Lasers
- •Clinical Application of Lasers
- •Condylomata Acuminata
- •Urolithiasis
- •Benign Prostatic Hyperplasia
- •Ureteral and Urethral Strictures
- •Conclusion
- •References
- •Introduction
- •The Prostatitis Syndromes
- •The Scope of the Problem
- •Category III CP/CPPS
- •The Goal of Treatment
- •Conservative Management
- •Drug Therapy
- •Antibiotics
- •Anti-inflammatories
- •Alpha blockers
- •Hormone Therapies
- •Phytotherapies
- •Analgesics, muscle relaxants and neuromodulators
- •Surgery
- •A Practical Management Plan
- •References
- •Orchitis
- •Definition and Etiology
- •Clinical Signs and Symptoms
- •Diagnostic Evaluation
- •Treatment of Infectious Orchitis
- •Epididymitis
- •Definition and Etiology
- •Clinical Signs and Symptoms
- •Diagnostic Evaluation of Epididymitis
- •Treatment of Acute Epididymitis
- •Treatment of Chronic Epididymitis
- •Treatment of Spermatic Cord Torsion
- •Fournier’s Gangrene
- •Definition and Etiology
- •Risk Factors
- •Clinical Signs and Symptoms
- •Diagnostic Evaluation
- •Treatment
- •References
- •Fungal Infections
- •Candidiasis
- •Aspergillosis
- •Cryptococcosis
- •Blastomycosis
- •Coccidioidomycosis
- •Histoplasmosis
- •Radiographic Findings
- •Treatment
- •Tuberculosis
- •Clinical Manifestations
- •Diagnosis
- •Treatment
- •Schistosomiasis
- •Clinical Manifestations
- •Diagnosis
- •Treatment
- •Filariasis
- •Clinical Manifestations
- •Diagnosis
- •Treatment
- •Onchocerciasis
- •References
- •25: Sexually Transmitted Infections
- •Introduction
- •STIs Associated with Genital Ulcers
- •Herpes Simplex Virus
- •Diagnosis
- •Treatment
- •Chancroid
- •Diagnosis
- •Treatment
- •Syphilis
- •Diagnosis
- •Treatment
- •Lymphogranuloma Venereum
- •Diagnosis
- •Treatment
- •Chlamydia
- •Diagnosis
- •Treatment
- •Gonorrhea
- •Diagnosis
- •Treatment
- •Trichomoniasis
- •Diagnosis
- •Treatment
- •Human Papilloma Virus
- •Diagnosis
- •Treatment
- •Scabies
- •Diagnosis
- •Treatment
- •References
- •26: Hematuria: Evaluation and Management
- •Introduction
- •Classification of Hematuria
- •Macroscopic Hematuria
- •Microscopic Hematuria
- •Dipstick Hematuria
- •Pseudohematuria
- •Factitious Hematuria
- •Menstruation
- •Aetiology
- •Malignancy
- •Urinary Calculi
- •Infection and Inflammation
- •Benign Prostatic Hyperplasia
- •Trauma
- •Drugs
- •Nephrological Causes
- •Assessment
- •History
- •Examination
- •Investigations
- •Dipstick Urinalysis
- •Cytology
- •Molecular Tests
- •Blood Tests
- •Flexible Cystoscopy
- •Upper Urinary Tract Evaluation
- •Renal USS
- •KUB Abdominal X-Ray
- •Intravenous Urography (IVU)
- •Computed Tomography (CT)
- •Retrograde Urogram Studies
- •Magnetic Resonance Imaging (MRI)
- •Additional Tests and Renal Biopsy
- •Intractable Hematuria
- •Loin Pain Hematuria Syndrome
- •References
- •27: Benign Prostatic Hyperplasia (BPH)
- •Historical Background
- •Pathophysiology
- •Patient Assessment
- •Treatment of BPH
- •Watchful Waiting
- •Drug Therapy
- •Interventional Therapies
- •Conclusions
- •References
- •28: Practical Guidelines for the Treatment of Erectile Dysfunction and Peyronie´s Disease
- •Erectile Dysfunction
- •Introduction
- •Diagnosis
- •Basic Evaluation
- •Cardiovascular System and Sexual Activity
- •Optional Tests
- •Treatment
- •Medical Treatment
- •Oral Agents
- •Phosphodiesterase Type 5 (PDE 5) Inhibitors
- •Nonresponders to PDE5 Inhibitors
- •Apomorphine SL
- •Yohimbine
- •Intracavernosal and Intraurethral Therapy
- •Intracavernosal Injection (ICI) Therapy
- •Intraurethral Therapy
- •Vacuum Constriction Devices
- •Surgical Therapy
- •Conclusion
- •Peyronie´s Disease (PD)
- •Introduction
- •Oral Drug Therapy
- •Intralesional Drug Therapy
- •Iontophoresis
- •Radiation Therapy
- •Surgical Therapy
- •References
- •29: Premature Ejaculation
- •Introduction
- •Epidemiology
- •Defining Premature Ejaculation
- •Voluntary Control
- •Sexual Satisfaction
- •Distress
- •Psychosexual Counseling
- •Pharmacological Treatment
- •On-Demand Treatment with Tramadol
- •Topical Anesthetics
- •Phosphodiesterase Inhibitors
- •Surgery
- •Conclusion
- •References
- •30: The Role of Interventional Management for Urinary Tract Calculi
- •Contraindications to ESWL
- •Complications of ESWL
- •PCNL Access
- •Instrumentation for PCNL
- •Nephrostomy Drains Post PCNL
- •Contraindications to PCNL
- •Complications of PCNL
- •Semirigid Ureteroscopy
- •Flexible Ureteroscopy
- •Electrohydraulic Lithotripsy (EHL)
- •Ultrasound
- •Ballistic Lithotripsy
- •Laser Lithotripsy
- •Ureteric Stents
- •Staghorn Calculi
- •Lower Pole Stones
- •Horseshoe Kidneys and Stones
- •Calyceal Diverticula Stones
- •Stones and PUJ Obstruction
- •Treatment of Ureteric Colic
- •Medical Expulsive Therapy (MET)
- •Intervention for Ureteric Stones
- •Stones in Pregnancy
- •Morbid Obesity
- •References
- •Anatomy and Function
- •Pathophysiology
- •Management
- •Optical Urethrotomy/Dilatation
- •Urethral Stents
- •Preoperative Assessment
- •Urethroplasty
- •Anastomotic Urethroplasty
- •Substitution Urethroplasty
- •Grafts Versus Flaps
- •Oral Mucosal Grafts
- •Tissue Engineering
- •Graft Position
- •Conclusion
- •References
- •32: Urinary Incontinence
- •Epidemiology and Risk Factors
- •Pathophysiology
- •Urge Incontinence
- •Conservative Treatments
- •Pharmacotherapy
- •Invasive/ Surgical Therapies
- •Stress Urinary Incontinence
- •Male SUI Therapies
- •Female SUI Therapies
- •Mixed Urinary Incontinence
- •Conclusions
- •References
- •33: Neurogenic Bladder
- •Introduction
- •Examination and Diagnostic Tests
- •History and Physical Examination
- •Imaging
- •Urodynamics (UDS)
- •Evoked Potentials
- •Classifications
- •Somatic Pathways
- •Brain Lesions
- •Cerebrovascular Accident (CVA)
- •Parkinson’s Disease (PD)
- •Multiple Sclerosis
- •Huntington’s Disease
- •Dementias
- •Normal Pressure Hydrocephalus (NPH)
- •Tumors
- •Psychiatric Disorders
- •Spinal Lesions and Pathology
- •Intervertebral Disk Prolapse
- •Spinal Cord Injury (SCI)
- •Transverse Myelitis
- •Peripheral Neuropathies
- •Metabolic Neuropathies
- •Pelvic Surgery
- •Treatment
- •Summary
- •References
- •34: Pelvic Prolapse
- •Introduction
- •Epidemiology
- •Anatomy and Pathophysiology
- •Evaluation and Diagnosis
- •Outcome Measures
- •Imaging
- •Urodynamics
- •Indications for Management
- •Biosynthetics
- •Surgical Management
- •Anterior Compartment Repair
- •Uterine/Apical Prolapse
- •Enterocele Repair
- •Conclusion
- •References
- •35: Urinary Tract Fistula
- •Introduction
- •Urogynecologic Fistula
- •Vesicovaginal Fistula
- •Etiology and Risk Factors
- •Clinical Factors
- •Evaluation and Diagnosis
- •Pelvic Examination
- •Cystoscopy
- •Imaging
- •Treatment
- •Conservative Management
- •Surgical Management
- •Urethrovaginal Fistula
- •Etiology and Presentation
- •Diagnosis and Management
- •Ureterovaginal Fistula
- •Etiology and Presentation
- •Diagnosis and Management
- •Vesicouterine Fistula
- •Etiology and Presentation
- •Diagnosis and Management
- •Uro-Enteric Fistula
- •Vesicoenteric Fistula
- •Pyeloenteric Fistula
- •Urethrorectal Fistula
- •References
- •36: Urologic Trauma
- •Introduction
- •Kidney
- •Expectant Management
- •Endovascular Therapy
- •Operative Intervention
- •Operative Management: Follow-up
- •Reno-Vascular Injuries
- •Pediatric Renal Injuries
- •Adrenal
- •Ureter
- •Diagnosis
- •Treatment
- •Delayed Diagnosis
- •Bladder and Posterior Urethra
- •Bladder Injuries: Initial Management
- •Bladder Injuries: Formal Repair
- •Anterior Urethral Trauma
- •Fractured Penis
- •Penile Amputation
- •Scrotal and Testicular Trauma
- •Imaging
- •CT-IVP (CT with Delayed Images)
- •Technique
- •Cystogram
- •Technique
- •Retrograde Urethrogram (RUG)
- •Technique
- •Retrograde Pyelogram (RPG)
- •Technique
- •One-Shot IVP
- •Technique
- •References
- •37: Bladder Cancer
- •Who Should Be Investigated?
- •Epidemiology
- •Risk Factors
- •Role of Screening
- •Signs and Symptoms
- •Imaging
- •Cystoscopy
- •Urine Tests
- •PDD-Assisted TUR
- •Pathology
- •NMIBC and Risk Groups
- •Intravesical Chemotherapy
- •Intravesical Immunotherapy
- •Immediate Cystectomy and CIS
- •Radical Cystectomy with Pelvic Lymph Node Dissection
- •sexual function-preserving techniques
- •Bladder-Preservation Treatments
- •Neoadjuvant Chemotherapy
- •Adjuvant Chemotherapy
- •Preoperative Radiotherapy
- •Follow-up After TUR in NMIBC
- •References
- •38: Prostate Cancer
- •Introduction
- •Epidemiology
- •Race
- •Geographic Variation
- •Risk Factors and Prevention
- •Family History
- •Diet and Lifestyle
- •Prevention
- •Screening and Diagnosis
- •Current Screening Recommendations
- •Biopsy
- •Pathology
- •Prognosis
- •Treatment of Prostate Cancer
- •Treatment for Localized Prostate Cancer (T1, T2)
- •Radical Prostatectomy
- •EBRT
- •IMRT
- •Brachytherapy
- •Treatment for Locally Advanced Prostate Cancer (T3, T4)
- •EBRT with ADT
- •Radical Prostatectomy
- •Androgen-Deprivation Therapy
- •Summary
- •References
- •39: The Management of Testis Cancer
- •Presentation and Diagnosis
- •Serum Tumor Markers
- •Primary Surgery
- •Testis Preserving Surgery
- •Risk Stratification
- •Surveillance Versus Primary RPLND
- •Primary RPLND
- •Adjuvant Treatment for High Risk
- •Clinical Stage 1 Seminoma
- •Risk-Stratified Adjuvant Treatment
- •Adjuvant Radiotherapy
- •Adjuvant Low Dose Chemotherapy
- •Primary Combination Chemotherapy
- •Late Toxicity
- •Salvage Strategies
- •Conclusion
- •References
- •Index

93
PrinciPlEs of BactErial Urinary tract infEctions and antimicroBials
Epidemiological Breakpoints
The epidemiological breakpoints are categorized into wild-type and non-wild-type microorganisms by applying the appropriate cut-off value in a defined phenotypic test system. Wild-type and non-wild type microorganisms may or may not respond clinically to antimicrobial treatment. These cut-off values will not be altered by changing circumstances.
•Wild-type: A microorganism is defined as wildtype for a species by the absence of acquired and mutational resistance mechanisms to the drug in question.
•Microbiological resistance-non-wild type: A microorganism is defined as non-wild type for a species by the presence of an acquired or mutational resistance mechanism to the drug in question.
Clinical Breakpoints
The clinical breakpoints are categorized into susceptible,intermediate,and resistant by applying the appropriate breakpoint in a defined phenotypic test system. These clinical breakpoints may be altered with legitimate changes in circumstances, such as new pharmacological data on antimicrobial substances.
•Clinically susceptible (S): A microorganism is defined as susceptible by a level of antimicrobial activity associated with a high likelihood of therapeutic success.
•Clinically intermediate (I): A microorganism is defined as intermediate by a level of antimicrobial agent activity associated with uncertain therapeutic effect. It implies that an infection due to the isolate may be appropriately treated in body sites where the drugs are physically concentrated or when a high dosage of drug can be used; it also indicates a buffer zone that should prevent small, uncontrolled, technical factors from causing major discrepancies in interpretations.
•Clinically resistant (R): A microorganism is defined as resistant by a level of antimicrobial activity associated with a high likelihood of therapeutic failure.
The establishment of clinical breakpoints implies the assessment of pharmacodynamic and pharmacokinetic parameters.
Pharmacodynamic Parameters
Pharmacodynamic parameters are those that describe the action of an antimicrobial at the bacterial cell. Several laboratory tests have been implemented to assess phenotypical pharmacodynamic parameters in vitro and thus predict the effectiveness of an antimicrobial therapy in vivo.
The primary aim of laboratory testing with antimicrobials is to predict therapeutic success in the individual patient treated. The most frequently used tests were designed to quantitate the lowest concentration of an antimicrobial that inhibits visible in vitro growth of the microbe, which is called the minimal inhibitory concentration (MIC). The MIC can be assessed by different tests, the most frequently used tests are the brothand agar dilution tests. The disk diffusion procedure is a simplified method to approximate the MIC. The MIC is a parameter defined to predict therapeutic success, at least in an immunocompetent environment. The factors that determine the outcome of an infection are, however, complex and incompletely addressed by in vitro tests. A variety of factors can influence the efficacy of antimicrobials in vivo: Aminoglycosides or fluoroquinolones lose their activity in acidic pH as frequently found in urine or at sites of purulent infection. The concentrations of certain cations, such as Ca2+ or Mg2+, which is extensively varying in urine, also influence the activity of aminoglycosides or fluoroquinolones in UTI. The MIC has therefore to be interpreted with caution.
Theminimalbactericidalconcentration(MBC) is defined as the lowest concentration of an antimicrobial that kills 99.9% of the inoculum. This test is not used in clinical routine with the exception of certain questions, such as treatment of infections in compartments deprived of the immune system, such as endocarditis, meningitis, or neutropenic situations. The MBC can also be used in situations where the medium to be tested is turbid, such as urine, where the inhibition of growth cannot be assessed.
Because of the worldwide increase of antibiotic resistance, a secondary aim in the treatment
94
Practical Urology: EssEntial PrinciPlEs and PracticE
of infections has been defined for antimicrobial substances. Especially in the development of new antibiotics, substances with a low potential to cause emergence of antibiotic resistance are selected nowadays.One parameter that describes the probability of an antimicrobial substance is the mutant preventive concentration (MPC). The MPC is defined as the concentration needed to inhibit the emergence of resistant mutants among 1010 bacteria. The MPC might be a future parameter to assess antimicrobial substances and dosing.
Pharmacokinetic Parameters
The pharmacokinetic parameters describe the absorption, distribution, and elimination of an antimicrobial substance, which also includes metabolization of a substance. For simplification, the pharmacokinetic parameters are usually assessed in the blood. However, the pivotal target is the effective antibiotic concentration at the site of infection, which in case of urinary tract infections is the urine and the tissues of the urogenital organs (kidney, bladder, prostate). Additionally, modifying factors, such as protein binding, will influence the concentrations at the site of infection. Pharmacokinetics can also vary remarkably within the population and especially between the patients being the targets treated. Therefore, mathematical models such as the Monte-Carlo simulations are nowadays applied to transfer limited pharmacokinetic data from clinical studies to large population collectives and better reflect the interindividual variations.
Pharmacokinetic/Pharmacodynamic
Correlations
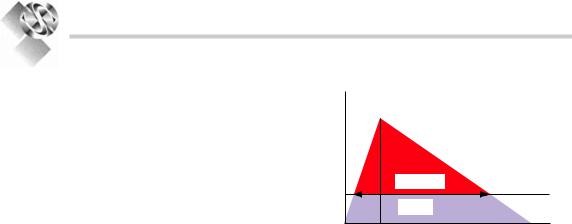
The effect of an antimicrobial substance over the time at the site of infection is the most important pharmacological parameter reflecting therapeutic success. This aspect can be assessed in an integrated evaluation of the pharmacokinetic and pharmacodynamic parameters. In this regard pharmacokinetic parameters are often correlated with the MIC as the pharmacodynamic parameter (Fig. 6.1). Employing those evaluations it became evident that drug classes behave differently toward the pathogens at which they are directed:
Conc.
(mg/L) Cmax
AUC/MIC |
MIC |
T>MIC |
Time (t)
Figure 6.1. association of pharmacokinetic and pharmacodynamic parameters in serum. Cmax maximal concentration, MIC minimal inhibitory concentration, AUC/MIC area under the curve (aUc) over mic, T>MIC time above mic.
b-Lactam agents have their rate of killing maximized at a low multiple of the MIC, because the effect of b-lactams is dependent on their binding to the b-lactam-binding protein within the bacterial cell. The antibacterial effect only appears if a substantial portion of the binding proteins is already occupied by the b-lactam agent. At increasing drug concentrations, the effect quickly maximizes. Therefore, achieving higher drug concentrations does not result in greater killing. For b-lactam agents the best therapeutic results are obtained by using smaller doses more frequently during the day.8
Other antibiotic substances such as aminoglycosides or fluoroquinolones exhibit a concentration dependent post-antibiotic effect. For example,after the removal of the antibiotic there exists still an inhibitory effect on the bacterial cells. This inhibitory effect is greater the higher the initial concentration is. Therefore, for best therapeutic results those antibiotics are usually administered once or twice per day with high doses in order to achieve high peak concentrations (Cmax) or a high area under the concentration time curve (AUC).
Bacterial Spectrum and
Antimicrobial Resistance
Patterns
The above mentioned aspects lead to a selection of antibiotic substances for the individual indication, such as UTI. In the next step a further selection is carried forward based upon the

95
PrinciPlEs of BactErial Urinary tract infEctions and antimicroBials
bacterial spectrum and the current antimicrobial resistance patterns. This is modified over the time depending on the development of bacterial resistance.
In uncomplicated UTI E. coli is the most common pathogen, typically being isolated from approximately 80% of outpatients with acute uncomplicated cystitis across the various regions of the world.2,9-11
A recent surveillance study, the ARESC (Antimicrobial Resistance Epidemiology Survey on Cystitis) project exclusively investigated female patients with uncomplicated cystitis.12 The results of this study showed that antibiotic substances classically used for the treatment of uncomplicated cystitis, such as TMP/SMX, fluoroquinolones or aminopenicillins, lose their effectiveness due to increasing resistance. Substances with high susceptibility rates were fosfomycin tromethamine, nitrofurantoin, or
pivmecillinam.
In uncomplicated pyelonephritis, the identical resistance developments should be expected. For treatment, fluoroquinolones or group 3a cephalosporines might be suitable substances.
In complicated and nosocomially acquired UTI, Gram-negative species account for approximately 60–80% of the bacterial spectrum and comprises E. coli, followed by Klebsiella spp.,
Pseudomonas spp., Proteus spp., Enterobacter spp., and Citrobacter spp. The Gram-positive pathogens account for about 20–40% of the spectrum and comprise enterococci and staphylococci.13-18
Nosocomial uropathogens are frequently subject to antibiotic pressure and cross-infection. Different species of uropathogens show distinct abilities to develop antibiotic resistance.
Surveillance studies such as the SENTRY-, ESGNIor PEP study, or a local urological surveillance study revealed that, considering the total bacterial spectrum investigated, in general the aminopenicillins (with b-lactamase inhibitors) showed resistance rates of approximately 60% (respectively 30%). TMP/SMX showed resistance rates between 22% and 45%. Resistance to ciprofloxacin was approximately 20–40%, to gentamicin 18–34%, to ceftazidime 13–28%, to piperacillin/ tazobactam 8–15%, to imipenem 7–14%. Resistance in enterococci to vancomycin was between 0% and 5%.13-18 In all the studies, increasing rates of antibiotic resistance were found with specific species like E. coli,
P. aeruginosa, Klebsiella spp., Enterobacter spp., enterococci, and coagulase negative staphylococci. Extended-spectrum b-lactamase producing E. coli and K. pneumoniae rapidly increase and may cause significant clinical problems in the treatment of UTI.19,20 Carbapenems fortunately still retained their activity in most of these uropathogens.
Election of an Antibiotic
Treatment
The choice of an antibiotic and its dosage for the treatment of an infection depends on all those described aspects and parameters. Additionally, nowadays the different propensity of the antibiotics to cause so called collateral damages is also considered. Collateral damages are defined as ecological adverse effects of antibiotic therapy, namely, the selection of drug-resistant organisms and the unwanted development of colonization or infection with multidrug-resistant organisms. Different antibiotic classes have significantly different propensities to cause collateral damage.Cephalosporin use,for example,has been linked to subsequent infection with vanco- mycin-resistant enterococci, extended-spectrum beta-lactamase-producing (ESBL) Klebsiella pneumoniae,beta-lactam-resistant Acinetobacter species, and Clostridium difficile. Quinolone use has been linked to infection with methicillinresistant Staphylococcus aureus(MRSA) and with increasingquinoloneresistanceinGram-negative bacilli, such as Pseudomonas aeruginosa.21
To incorporate these different aspects into a concept of antimicrobial chemotherapy of UTIs, for uncomplicated UTIs and especially uncomplicated cystitis, antimicrobials exclusively used for this indication are recommended. For complicated UTIs antibiotics with optimal activity in the urinary tract and high dosage are recommended.
Uncomplicated, Community
Acquired UTI
Ideal substances are those with high susceptibility rates, exclusively used for this indication, such as fosfomycin tromethamine, nitrofurantoin or pivmecillinam for the treatment of uncomplicated cystitis (Table 6.1).
