
PS-2020a / part17
.pdf
|
DICOM PS3.17 2020a - Explanatory Information |
Page 781 |
|
Node |
Code Meaning of Concept Name Code Meaning or Example |
TID |
|
|
|
Value |
|
1.12.3.2.3.1 |
Drug administered |
Ketamine |
TID 8131 |
|
|
|
CID 623 |
1.12.3.2.3.2 |
Medication Type |
General anesthetic |
TID 8131 |
|
|
|
CID 621 |
1.12.3.2.3.2 |
Dosage |
nn mg |
TID 8131 |
1.12.3.2.4 |
Mixture |
|
TID 8131 |
1.12.3.2.4.1 |
Drug administered |
Medetomidine |
TID 8131 |
|
|
|
CID 623 |
1.12.3.2.4.2 |
Medication Type |
General anesthetic |
TID 8131 |
|
|
|
CID 621 |
1.12.3.2.4.2 |
Dosage |
nn mg |
TID 8131 |
... |
... |
... |
... |
YYY.1.2 Example of exogenous substance administration to encode tumor cell line
Onlytheexogenoussubstanceinformationisincludedinthisexampleandcontentdescribinganimalhandling,anesthesiainformation, etc. is excluded for clarity. Indeed, given the optionality of the other content, it would be possible to create an Acquisition Context SR instance that describes only the exogenous substance information and nothing else.
The content tree structure would resemble:
Node |
Code Meaning of Concept Name |
Code Meaning or Example |
TID |
|
|
|
Value |
|
|
1 |
Preclinical Small Animal Imaging |
|
TID 8101 |
|
|
Acquisition Context |
|
|
|
1.1 |
Language of Content Item and |
English |
TID 1204 |
|
|
Descendants |
|
|
|
1.1.2 |
Country of Language |
United States |
TID 1204 |
|
1.2 |
Person Observer Name |
Doe^Jane |
TID 1003 |
|
... |
... |
... |
... |
|
1.n |
Exogenous substance |
|
TID 8182 |
|
1.n.1 |
Tumor Graft |
Adenocarcinoma |
TID 8182 |
|
|
|
|
CID 637 |
|
|
|
|
CID 639 |
|
1.n.1.1 |
Age Started |
6 week |
TID 8182 |
|
|
|
|
CID 7456 |
|
1.n.1.2 |
DateTime Started |
yyyymmddhhss |
TID 8182 |
|
1.n.1.3 |
Brand Name |
MDA-MB-468 |
TID 8182 |
|
1.n.1.4 |
Dosage |
10E6 {cells} |
TID 8182 |
|
|
|
|
CID 6092 |
|
- Standard -

Page 782 |
DICOM PS3.17 2020a - Explanatory Information |
|
|
|
Node |
Code Meaning of Concept Name |
Code Meaning or Example |
TID |
|
|
|
Value |
|
|
1.n.1.5 |
Relative dose frequency |
Single event |
TID 8182 |
|
|
|
|
CID 6094 |
|
|
|
|
CID 6091 |
|
1.n.1.6 |
Route of Administration |
Subcutaneous route |
TID 8182 |
|
|
|
|
CID 11 |
|
1.n.1.6.1 |
Site of |
Flank |
TID 8182 |
|
|
|
|
CID 644 |
|
1.n.1.6.1.1 |
Laterality |
Left |
TID 8182 |
|
|
|
|
CID 244 |
|
1.n.1.7 |
Tissue of origin |
Breast |
TID 8182 |
|
|
|
|
CID 645 |
|
1.n.1.8 |
Taxonomic rank of origin |
homo sapiens |
TID 8182 |
|
|
|
|
CID 7454 |
|
YYY.1.3 Informative References
YYY.1.3.1 Method Descriptions
[Stout et al 2013] Molecular Imaging. Stout D, Berr SS, LeBlanc A, Kalen JD, Osborne D, Price J, Schiffer W, Kuntner C, and Wall J. 2013. 12. 7. 1-15. “Guidance for Methods Descriptions Used in Preclinical Imaging Papers”. http://journals.sagepub.com/ doi/pdf/10.2310/7290.2013.00055 .
[David et al 2013a] Comparative Medicine. David JM, Chatziioannou AF, Taschereau R, Wang H, and Stout DB. 2013. 63. 5. 386–91. “The Hidden Cost of Housing Practices: Using Noninvasive Imaging to Quantify the Metabolic Demands of Chronic Cold Stress of Laboratory Mice”. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3796748/ .
[David et al 2013b] Journal of the American Association for Laboratory Animal Science. David JM, Knowles S, Lamkin DM, and Stout DB. 2013. 52. 6. 738–44. “Individually Ventilated Cages Impose Cold Stress on Laboratory Mice: A Source of Systemic Ex- perimental Variability”. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3838608/ .
[Rosenbaum et al 2009] Journal of the American Association for Laboratory Animal Science. Rosenbaum MD. 2009. 48. 6. 763–73. “EffectsofCage-ChangeFrequencyandBeddingVolumeonMiceandTheirMicroenvironment”. http://www.ncbi.nlm.nih.gov/ pmc/articles/PMC2786931/ .
[Fueger et al 2006] Journal of Nuclear Medicine. Fueger BJ. 2006. 47. 6. 999–1006. “Impact of Animal Handling on the Results of 18F-FDG PET Studies in Mice”. http://jnm.snmjournals.org/content/47/6/999 .
[Dandekar et al 2007] Journal of Nuclear Medicine. Dandekar M. 2007. 48. 4. 602–7. “Reproducibility of 18F-FDG microPET Studies in Mouse Tumor Xenografts”. 10.2967/jnumed.106.036608. http://jnm.snmjournals.org/content/48/4/602 .
[Lee et al 2005] Journal of Nuclear Medicine. Lee KH. 2005. 46. 9. 1531–36. “Effects of Anesthetic Agents and Fasting Duration on 18F-FDG Biodistribution and Insulin Levels in Tumor-Bearing Mice”. http://jnm.snmjournals.org/content/46/9/1531 .
[Balcombe et al 2004] Journal of the American Association for Laboratory Animal Science. Balcombe JP. 2004. 43. 6. 42–51. “Laboratory Routines Cause Animal Stress”. .
[Van der Meer et al 2004] Journal of the American Association for Laboratory Animal Science. Van der Meer E. 2004. 38. 4. 376–83. “Short-termeffectsofadisturbedlight–darkcycleandenvironmentalenrichmentonaggressionandstress-relatedparameters in male mice”. http://www.animalexperiments.info/resources/Studies/Animal-impacts/Stress.-Balcombe-et-al-2004./ Stress-Balcombe-et-al-2004.pdf .
- Standard -

DICOM PS3.17 2020a - Explanatory Information |
Page 783 |
[Tabata et al 1998] Laboratory Animals. Tabata H. 1998. 32. 2. 143–48. “Comparison of Effects of Restraint, Cage Transportation, Anaesthesia and Repeated Bleeding on Plasma Glucose Levels between Mice and Rats”. 10.1258/002367798780599983. http://lan.sagepub.com/content/32/2/143 .
- Standard -

Page 784 |
DICOM PS3.17 2020a - Explanatory Information |
- Standard -
DICOM PS3.17 2020a - Explanatory Information |
Page 785 |
ZZZ Content Assessment (Informative)
The following use cases exemplify the use of Content Assessment Results IOD.
ZZZ.1 RT Plan Treatment Assessment Use Case
ARTPlanSOPInstanceissentfromaTreatmentPlanningSystem(TPS)toaQualityAssurance(QA)ApplicationandtotheTreatment Management System (TMS). The TMS de-composes the content for internal storage. At the time of treatment the TMS re-composes the Instance and sends it to the operator console of the linear accelerator. However, during re-composition an error occurs and one jaw specification is omitted from the recomposed Instance and the Beam Dose in the Fraction Scheme Module is set to 0.0.
The operator console requests the QA Application to perform an assessment to compare the copy of the Instance received from the operator console with the copy of the Instance received earlier from the TPS. The QA Application retrieves the Instance from the op- eratorconsole.TheQAApplicationalsoperformsanassessmentbyre-calculationofthedosimetricparametersintheassessedplan. Although the Beam Meterset in the assessed plan (from the operator console) is the same as the Beam Meterset in the comparison plan(fromtheTPS),theBeamMetersetre-calculatedbytheQAApplicationisdifferentduetothemissingjaw.Furtheronitisdetected, that all Beam Dose values have the value 0.0.
Beam Meterset for the current treatment device in this example is expressed in Monitor Units.
Table ZZZ.1-1. Content Assessment Results Module Example of a RT Plan Treatment Assessment
Nesting |
Attribute Name |
Tag |
VR |
Value |
|
Assessment Label |
(0082,0023) |
LO |
Pre-Treatment Assessment of |
|
|
|
|
Fraction 7 |
|
Assessment Type Code Sequence |
(0082,0021) |
SQ |
|
%item |
|
|
|
|
|
>Code Value |
(0008,0100) |
SH |
121373 |
|
>Coding Scheme Designator |
(0008,0102) |
SH |
DCM |
|
>Code Meaning |
(0008,0104) |
LO |
RT Pre-Treatment Consistency |
|
|
|
|
Check |
%enditem |
|
|
|
|
%endsq |
(Assessment Type Code Sequence) |
|
|
|
|
Assessment Set ID |
(0082,0016) |
LO |
ID12345 |
|
Assessment Requester Sequence |
(0082,0017) |
SQ |
|
%item |
|
|
|
|
|
>Observer Type |
(0040,A084) |
CS |
DEV |
|
>Station Name |
(0008,1010) |
SH |
CONSOLE 1 |
|
>Device UID |
(0018,1002) |
UI |
1.2.3.4.5.6.7.8.9.10 |
|
>Manufacturer |
(0008,0070) |
LO |
Fancy Linac Inc. |
|
>Manufacturer's Model Name |
(0008,1090) |
LO |
Linear Accelerator Console |
|
>Institution Name |
(0008,0080) |
LO |
RT Clinic 1 |
|
>Institution Code Sequence |
(0008,0082) |
SQ |
|
%item |
|
|
|
|
|
>>Code Value |
(0080,0100) |
SH |
Clinic1 |
|
>>Coding Scheme Designator |
(0008,0102) |
SH |
99MyCounty |
|
>>Code Meaning |
(0008,0104) |
LO |
RT Clinic 1 |
%enditem |
|
|
|
|
- Standard -

Page 786 |
DICOM PS3.17 2020a - Explanatory Information |
|
||
Nesting |
Attribute Name |
Tag |
VR |
Value |
%endsq |
(>Institution Code Sequence) |
|
|
|
%enditem |
|
|
|
|
%endsq |
(Assessment Requester Sequence) |
|
|
|
|
Assessed SOP Instance Sequence |
(0082,0004) |
SQ |
|
%item |
|
|
|
|
|
>Referenced SOP Class UID |
(0008,1150) |
UI |
1.2.840.10008.5.1.4.1.1.481.5 |
|
|
|
|
(RT Plan Storage) |
|
>Referenced SOP Instance UID |
(0008,1155) |
UI |
1.2.3.4.5.300 |
%enditem |
|
|
|
|
|
>ReferencedComparisonSOPInstance |
(0082,0005) |
SQ |
|
|
Sequence |
|
|
|
%item |
|
|
|
|
|
>>Referenced SOP Class UID |
(0008,1150) |
UI |
1.2.840.10008.5.1.4.1.1.481.5 |
|
|
|
|
(RT Plan Storage) |
|
>>Referenced SOP Instance UID |
(0008,1155) |
UI |
1.2.3.4.5.300 |
%enditem |
|
|
|
|
%endsq |
(>ReferencedComparisonSOPInstance |
|
|
|
|
Sequence) |
|
|
|
%enditem |
|
|
|
|
%endsq |
(Assessed SOP Instance Sequence) |
|
|
|
|
Assessment Summary |
(0082,0001) |
CS |
FAILED |
|
Assessment Summary Description |
(0082,0003) |
UT |
Plan Checker result: Failed! |
|
|
|
|
The assessed RT Plan does not |
|
|
|
|
match the reference RT Plan it is |
|
|
|
|
compared to. One or more |
|
|
|
|
relevant Attributes are not equal. |
|
|
|
|
Monitor Unit values and Beam |
|
|
|
|
Doseshaveunreasonablevalues. |
|
Number of Assessment Observations |
(0082,0006) |
UL |
3 |
|
Assessment Observations Sequence |
(0082,0007) |
SQ |
|
%item |
|
|
|
|
|
>Observation Significance |
(0082,0008) |
CS |
MAJOR |
|
>Observation Basis Code Sequence |
(0082,0022) |
SQ |
|
%item |
|
|
|
|
|
>>Code Value |
(0008,0100) |
SH |
121375 |
|
>>Coding Scheme Designator |
(0008,0102) |
SH |
DCM |
|
>>Code Meaning |
(0008,0104) |
LO |
Assessment By Comparison |
%enditem |
|
|
|
|
%endsq |
(>Assessment Basis Code Sequence) |
|
|
|
|
>Observation Description |
(0082,000A) |
UT |
Attribute value of Leaf/Jaw |
|
|
|
|
Positions is not equal. |
|
>Structured Constraint Observation |
(0082,000C) |
SQ |
|
|
Sequence |
|
|
|
 %item
%item
- Standard -
|
DICOM PS3.17 2020a - Explanatory Information |
Page 787 |
||
Nesting |
Attribute Name |
Tag |
VR |
Value |
|
>>Selector Attribute Name |
(0082,0018) |
LO |
Leaf/Jaw Positions |
|
>>Selector Attribute VR |
(0072,0050) |
CS |
DS |
|
>>Selector Attribute |
(0072,0026) |
AT |
300A011C |
|
>>Selector Value Number |
(0072,0028) ) |
US |
1 |
|
>>Selector Sequence Pointer |
(0072,0052) |
AT |
300A00B0\300A0111\300A011A |
|
>>Selector Sequence Pointer Items |
(0074,1057) |
IS |
1\2\2 |
|
>>Constraint Type |
(0082,0032) |
CS |
EQUAL |
|
>>Constraint Violation Significance |
(0082,0036) |
CS |
FAILURE |
|
>>Constraint Value Sequence |
(0082,0034) |
SQ |
|
%item |
|
|
|
|
|
>>>Selector DS Value |
(0072,0072) |
DS |
-75.000\75.000 |
%enditem |
|
|
|
|
%endsq |
(>>Constraint Value Sequence) |
|
|
|
|
>>Assessed Attribute Value Sequence |
(0082,0010) |
SQ |
|
%item |
|
|
|
|
|
>>>Selector DS Value |
(0072,0072) |
DS |
-75.000 |
%enditem |
|
|
|
|
%endsq |
(>>AssessedAttributeValueSequence) |
|
|
|
%enditem |
|
|
|
|
%endsq |
(>Constraint Observation Sequence) |
|
|
|
%enditem |
|
|
|
|
%item |
|
|
|
|
|
>Observation Significance |
(0082,0008) |
CS |
MAJOR |
|
>Observation Basis Code Sequence |
(0082,0022) |
SQ |
|
%item |
|
|
|
|
|
>>Code Value |
(0008,0100) |
SH |
121376 |
|
>>Coding Scheme Designator |
(0008,0102) |
SH |
DCM |
|
>>Code Meaning |
(0008,0104) |
LO |
Assessment By Quality Rules |
%enditem |
|
|
|
|
%endsq |
(>Assessment Basis Code Sequence) |
|
|
|
|
>Observation Description |
(0082,000A) |
UT |
MonitorUnitsre-calculationfailed. |
|
|
|
|
The re-calculation of the beam |
|
|
|
|
meterset resulted in a different |
|
|
|
|
value (76MU) than the value in |
|
|
|
|
theassessedRTPlan.Thisvalue |
|
|
|
|
is outside the tolerance of |
|
|
|
|
reasonable differences |
|
|
|
|
acceptable on re-calculation. |
|
>Structured Constraint Observation |
(0082,000C) |
SQ |
|
|
Sequence |
|
|
|
%item |
|
|
|
|
|
>>Selector Attribute Name |
(0082,0018) |
LO |
Beam Meterset |
|
>>Selector Attribute VR |
(0072,0050) |
CS |
DS |
|
>>Selector Attribute |
(0072,0026) |
AT |
300A0086 |
- Standard -
Page 788 |
DICOM PS3.17 2020a - Explanatory Information |
|
||
Nesting |
Attribute Name |
Tag |
VR |
Value |
|
>>Selector Value Number |
(0072,0028) ) |
US |
1 |
|
>>Selector Sequence Pointer |
(0072,0052) |
AT |
300A0070\300C0004 |
|
>>Selector Sequence Pointer Items |
(0074,1057) |
IS |
1\1 |
|
>>Constraint Type |
(0082,0032) |
CS |
RANGE_INCL |
|
>>Constraint Violation Significance |
(0082,0036) |
CS |
FAILURE |
|
>>Constraint Value Sequence |
(0082,0034) |
SQ |
|
%item |
|
|
|
|
|
>>>Selector DS Value |
(0072,0072) |
DS |
68 |
%enditem |
|
|
|
|
%item |
|
|
|
|
|
>>>Selector DS Value |
(0072,0072) |
DS |
84 |
%enditem |
|
|
|
|
%endsq |
(>>Constraint Value Sequence) |
|
|
|
|
>>Assessed Attribute Value Sequence |
(0082,0010) |
SQ |
|
%item |
|
|
|
|
|
>>>Selector DS Value |
(0072,0072) |
DS |
108 |
%enditem |
|
|
|
|
%endsq |
(>>AssessedAttributeValueSequence) |
|
|
|
%enditem |
|
|
|
|
%endsq |
(>Constraint Observation Sequence) |
|
|
|
%enditem |
|
|
|
|
%item |
|
|
|
|
|
>Observation Significance |
(0082,0008) |
CS |
MODERATE |
|
>Observation Basis Code Sequence |
(0082,0022) |
SQ |
|
%item |
|
|
|
|
|
>>Code Value |
(0008,0100) |
SH |
121376 |
|
>>Coding Scheme Designator |
(0008,0102) |
SH |
DCM |
|
>>Code Meaning |
(0008,0104) |
LO |
Assessment By Quality Rules |
%enditem |
|
|
|
|
%endsq |
(>Observation Basis Code Sequence) |
|
|
|
|
>Observation Description |
(0082,000A) |
UT |
The Beam Dose value of all |
|
|
|
|
Beams is zero, but Beam |
|
|
|
|
Meterset is non-zero. |
%enditem |
|
|
|
|
%endsq |
(Assessment Observations Sequence) |
|
|
|
- Standard -
DICOM PS3.17 2020a - Explanatory Information |
Page 789 |
AAAA Protocol Storage Examples and Concepts (informative)
The following examples are provided to illustrate the usage of the CT Defined and Performed Procedure Protocol IODs. They do NOT represent recommended scanning practice. In some cases they have been influenced by published protocols, but the examples here may not fully encode those published protocols and no attempt has been made to keep them up-to-date.
AAAA.1 Protocol Storage Concepts
AAAA.1.1 Use Cases
Theprimaryapplications(usecases)consideredduringthedevelopmentoftheCTProcedureProtocolStorageIODswerethefollowing:
•Managing protocols within a site for consistency and dose management (Using Defined Protocols)
•Recording protocol details for a performed study so the same or similar values can be used when performing followup or repeat studies, especially for oncology which does comparative measurements (Using Performed Protocols)
•Vendor troubleshooting image quality issues that may be due to poor protocol/technique (Using Performed Protocols, Defined Protocols)
•Distributing departmental, "best practice" or reference protocols (such as AAPM) to modality systems (Using Defined Protocols)
•Backing up protocols from a modality to PACS or removable media (e.g., during system upgrades or replacement); most vendors have a proprietary method for doing this which would essentially become redundant when Protocol Management is implemented (Using Defined Protocols)
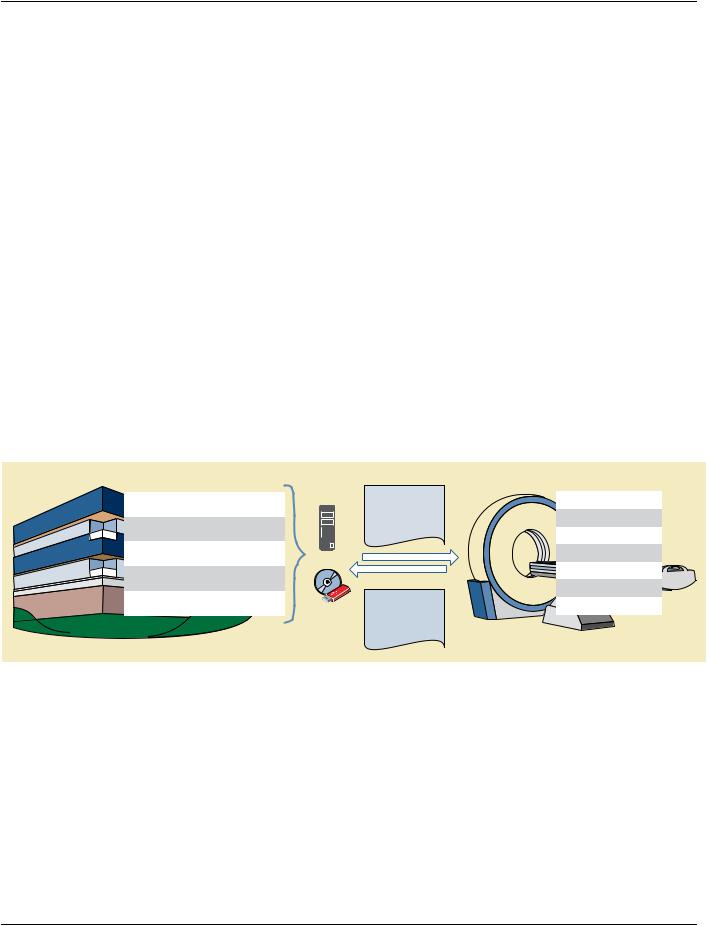
Sources |
|
Defined Protocol |
Applications |
|
|
Device = Scanomatic Series |
|
|
|
... |
Semi-auto Scan Setup |
Modality |
Prior Scan |
Pat. Pos. : Feet First / Supine |
|
Pat. Instruct. : Single Breath Hold |
|
||
|
|
|
|
|
|
/ Full Inspiration |
Prior Compatibility |
|
|
... |
|
Vendor |
Protocol Library |
kVp : 80~140 |
|
Slice Thickness : 1.0~2.5mm |
Patient Positioning |
||
|
|
Recon. Kernel : Std. |
|
|
|
... |
|
Clinical Trial |
Clinical Trial Protocol |
or... |
Sharing Best Practice |
|
|
|
|
Hospital |
Local Standards |
|
Protocol Backups |
Performed Protocol |
|
||
|
|
Acquisition Validation |
|
|
|
Device = Scanomatic Delta |
|
|
|
|
|
ACR/RSNA/AAPM |
Best Practice |
Version = 3.0.4 |
|
... |
Scan QA / Troubleshooting |
||
|
|
Pat. Pos. : Feet First / Supine |
|
|
|
|
|
|
|
Pat. Instruct. : Given |
|
|
|
... |
|
|
|
kVp : 120 |
|
|
|
Slice Thickness : 1.0mm |
|
|
|
Recon. Kernel : FC12 |
|
|
|
... |
|
Figure AAAA.1.1-1. Protocol Storage Use Cases
Additional potential applications include:
•Makingmoredetailedprotocolinformationavailabletorenderingorprocessingapplicationsthatwouldallowthemtoselectprocessing that corresponds to the acquisition protocol, to select parameters appropriate to the acquisition characteristics, and to select the right series to process/display (Using Performed Protocols)
•Improving imaging consistency in terms of repeatable technique, performance, quality and image charateristics; would benefit from associated image quality metrics and other physics work (Using Defined Protocols and Performed Protocols)
•Distributing clinical trial protocols (general purpose or scanner model specific) to participating sites (Using Defined Protocols)
•Recording protocol details for a performed study to submit with clinical trial images for technique validation (Using Performed Pro- tocols)
- Standard -

Page 790 |
DICOM PS3.17 2020a - Explanatory Information |
•Tracking/extracting details of Performed Protocol such as timestamps, execution sequence and technique for QA, data mining, etc. (Using Performed Protocols)
•Making more detailed protocol information available to radiologists reviewing a study and priors, or comparing similar studies of different patients (Using Performed Protocols)
AAAA.1.2 Workflow
Using non-Patient-specific Protocols
In most cases, the scanner uses any protocol details in the modality worklist item to present to the technologist a list of matching Defined Protocols on this scanner.
Preparing and executing Patient-specific Protocols
In the simplest form, this process could be driven with a combination of the Modality Worklist and Defined Protocols.
Radiologist at the RIS:
•Selects a patient procedure on the upcoming Modality Worklist
•Adds tech notes to the Worklist entry (e.g., "Use Defined Protocol X; Decrease parameter Y…")
Technologist at the modality:
•Selects the patient procedure on the upcoming Modality Worklist
•Reads the tech notes
•Selects the identified Defined Protocol and adjusts as described
Modality
•Executes procedure
•Stores the Performed Protocol to study folder on PACS
Radiologist
•(Optionally) Reviews the Performed Protocols
In special cases, the radiologist might attend the scan and modify the protocol directly on the console.
Note that the primary record of adjustments is the Performed Protocol object (which can be compared to the referenced Defined Protocol object).
A new Defined Protocol is not typically saved unless the intent is to have a new Defined Protocol available in the Library.
AAAA.2 Routine Adult Head Protocol
The examples in this Annex are intended to illustrate the encoding mechanisms of the DICOM CT Protocol Storage IODs, not to suggest particular values for clinical use. Further, these examples do not contain the many detailed Attributes one would expect from a fully executable defined protocol generated by a CT scanner, but they do demonstrate the usage of many common Attributes.
This section includes Defined Protocol examples of a Routine Adult Head Protocol for several different scanner models. The protocol is presented as adjusted by a fictitious Mercy Hospital from a reference protocol referenced in the Predecessor Protocol Sequence. AlthoughtheexamplesinthissectionwereoriginallyderivedfromprotocoldocumentspreviouslypublishedbytheAAPM,somevalues here were modified and are likely out of date. Parties interested in the current AAPM protocols are encouraged to visit ht- tp://www.aapm.org/pubs/CTProtocols/
- Standard -
