
- •Energy Saving Technologies Riga Technical University
- •Content
- •Introduction 10
- •1. Energy Saving Technologies in generation, conversion of electrical energy 11
- •Executive summary
- •Introduction
- •1.Energy Saving Technologies in generation, conversion of electrical energy
- •1.1.Cogeneration
- •1.1.1.Introduction
- •1.1.2.Performance indices of cogeneration systems
- •1.1.3.Types of cogeneration systems
- •Comparison of Fuel Cell Systems [12].
- •1.1.4.Distributed energy resources
- •Characteristics of cchp Systems [15].
- •References
- •1.2.Smart metering concept
- •1.2.1.Introduction
- •1.2.2.Communication concept of smart metering
- •1.2.2.1.Customer domain
- •1.2.2.2.Critical infrastructure energy domain
- •1.2.2.3.The utility business market communication domain
- •1.2.2.4.Third parties services - data analysis
- •Ip service provider’s domain
- •1.2.3.Wireless sensor networks in smart metering
- •1.2.3.1.Main characteristics of wireless sensor networks
- •1.2.3.2.Examples of application of wireless sensor networks
- •1.2.4.Security issues
- •1.2.5.The future of smart metering
- •1.3. Energy from biomass
- •1.3.1. Biomass resources
- •Yeld of Som Biomass Types [2].
- •Yield of Agricultural Residues [2].
- •1.3.1.Biomass conversion technologies
- •Characteristics of Solid Biofuels and their Effects.
- •Ultimate Analysis of Different Solid Biofuels (Dry Basis) [5, 6, 7].
- •Proximate Analysis of Solid Biofuels (Dry Basis) [5, 6, 7].
- •Characteristics of Compacted Biomass [2].
- •Higher Heating Value of Solid Biofuels [8, 9, 10].
- •Composition of Biomass Ash [5, 13].
- •Types of Biomass Furnaces [14].
- •Heat Capacity of Combustible Gas [17].
- •Contaminants in Combustible Gas: Problems and Cleanup Methods [17].
- •Syngas Quality Parameters.
- •Operating Parameters of Pyrolysis Processes.
- •1.4.Energy Storage
- •1.4.1.Introduction
- •1.4.2.Classification of energy storage technologies
- •Types of Energy Storage Technologies and Their Applications [2].
- •1.4.3.Characteristics of energy storage techniques
- •1.4.4.Direct electric storage
- •1.4.5.Electrochemical energy storage
- •1.4.6.Mechanical energy storage
- •The response time of sudden changes in electrical demand for power plants [5].
- •1.4.7.Thermal energy storage
- •Physical Properties of Sensible Energy Storage Media [7, 8]
- •Commercial Phase Change Materials which can be Used for Heat Storage in the Buildings [10].
- •Properties of Some Phase Change Materials Produced by eps Ltd, uk [11].
- •Properties of Some Phase Change Materials Produced by teap Energy, Australia [11].
- •Properties of some phase change materials (paraffins) produced by the Rubitherm GmbH Germany [11].
- •Chemical Storage Materials and Reactions [8].
- •Main Characteristics of Energy Storage Materials [8].
- •References
- •1.5.Waste heat recovery
- •1.5.1.Characteristics of waste heat
- •Sources of waste heat at high-temperature range [2].
- •Sources of Waste Heat at Medium-Temperature Range [2].
- •Sources of Waste Heat at Low-Temperature Range [2].
- •1.5.2.Waste heat recovery systems
- •Waste Heat Recovery Systems [3].
- •Heat Exchangers Characteristics.
- •References
- •1.6.Energy Saving Technologies of the Thermochemical Conversion of Biomass and lignocarbonaceous Waste
- •1.6.1.Introduction
- •1.6.2.Pyrolysis
- •1.6.3.1.2 Torrefaction
- •1.6.4.1.3 Fast pyrolysis
- •1.6.5.1.4. Flash and ultra-rapid pyrolysis
- •1.6.6.1.5. Solar driven pyrolysis
- •1.6 Pyrolizer types
- •1.7.Gasification
- •1.8. Poly-generation of heat, power and biofuel
- •1.9.Design of renewable energy systems for small (local) consumers - description of a software for design and examples of design exercises.
- •1.9.1.Introduction.
- •1.9.2.A software for design renewable energy systems.
- •1.9.3.Description of the polysun platform
- •1.9.3.1.Polysun modules
- •1.9.3.2.User Interface
- •1.9.3.2.1.Menu bar
- •1.9.3.2.2.Icon bar
- •1.9.3.2.3.Managing the project.
- •1.9.3.2.4.Project tools
- •1.9.4.Creating a project
- •1.9.4.1.Design steps of the simple solar system.
- •1.9.4.2.Design steps of the pv system.
- •1.9.5.Result analysis and reports
- •1.9.5.1.The results of simulation
- •1.9.5.2.Reports
- •1.9.6.Literature
- •Conclusion
- •2.Energy Saving Technologies in transmission, distribution of electrical energy Energy Cost and Power Loss Minimization in Distribution Networks with Distributed Generation
- •Introduction
- •2.1.Opf problem formulation for distribution networks
- •2.1.1.Objective function
- •2.1.2.Constraints
- •Dg units modeling for optimal power flow
- •Opf Solution Using Multi-objective Genetic Algorithm
- •Opf Solution Using Gravitational Search Algorithm
- •2.2.Dc transmission systems
- •3. Energy Saving Technologies: in industry
- •3.1. Electric Motors
- •3.2. Electrical Drives
- •3.1.Waste heat utilization technologies
- •Introduction
- •1 Sources of waste heat
- •2 Main definitions used for heat waste assessment
- •3 Using of waste heat for heating and hot water supply. Equipment for using of industrial waste heat
- •3.1 Closed-circuit schemes of waste heat utilization
- •3.2 Opened-circuit schemes of waste heat utilization
- •Indirect Contact Condensation Recover
- •4. Utilization of low-temperature heat waste
- •4.1 Heat pumps
- •Common types of industrial heat pumps
- •4.2 Applications of heat pumps in drying process
- •4.2.1 Closed-cycle mechanical heat pumps for lumber drying
- •4.2.2 Evaporation - open-cycle mechanical vapour compression (mvc) for sugar solution concentration
- •4.2.3 Thermo-compression for paper-dryer flash steam recovery
- •4.3 Heat pumps working fluids
- •5 Using of waste heat for power generation
- •5.1 The opportunity for waste heat to power generation
- •5.2 Applicable Technologies
- •5.3 Applications
- •Using of combustible waste
- •7 Economic efficiency analysis of heat waste utilization
- •4.Energy Saving Technologies: in public and private sector
- •4.1.Building: fundamental physical processes in buildings and building envelopes. Reduction of heat losses. Heating and conditioning. Heat pumps.
- •5.Supercapacitors
- •Viesturs Brazis
- •5.1.Supercapacitor energy storage
- •5.1.1.Introduction
- •5.1.2.Supercapacitor design
- •5.1.3.Supercapacitor energy storage systems
- •5.1.4.Simulation of supercapacitor energy storage system
- •5.1.5.Ess scaling
- •5.1.6.Conclusions
- •5.1.7.Tasks
- •References
- •5. Standartisation and legal bases on existing Energy Saving Technologies
- •5.2.Introduction
- •5.3.Legistlative base mandatory for eu Member states
- •5.4.Legistlative base non - mandatory for eu Member states
- •5.5.Eu supported actions for development of Energy Saving Technologies
- •5.6.Iso 50001 - Energy management
- •5.7.Conclusions
- •References
References
B. ZareNezhad, A. Aminian, Accurate prediction of the dew points of acidic combustion gases by using an artificial neural network model, Energy Conversion and Management 52 (2011), pp. 911–916.
C. Turner Wayne, S. Doty, Energy management handbook, 6th Edition, CRC Press, 2007.
M. Hasanuzzaman, N.A. Rahim, M. Hosenuzzaman, R. Saidur, I.M. Mahbubul, M.M. Rashid, Energy savings in the combustion based process heating in industrial sector, Renewable and Sustainable Energy Reviews 16 (2012), pp. 4527–4536.
D. Sjoding, Overview of Waste Heat Recovery for Power and Heat, Waste Heat Recovery for Power and Heat Workshop, Chicago, IL, September 29, 2010.
Waste Heat Recovery: Technology and Opportunities in U.S. Industry, U.S. department of Energy, 2008.
S. Kakaç, H. Liu, Heat Exchangers: Selection, Rating and Thermal Design, Second Edition, CRC Press, 2002.
Energy Efficiency Guide for Industry in Asia –www.energyefficiencyasia.org
www.industrialheating.com
W. Srimuang, P. Amatachaya, A review of the applications of heat pipe heat exchangers for heat recovery, Renewable and Sustainable Energy Reviews 16 (2012), pp. 4303– 4315.
Waste heat recovery (in Energy Management Series for Industry, Commerce and Institutions), Office of Energy Efficiency, Natural Resources Canada.
http://www.gocesco.com/index.html
1.6.Energy Saving Technologies of the Thermochemical Conversion of Biomass and lignocarbonaceous Waste
Executive summary
Chapter 1: Pyrolysis (pp.11-30), 10 figures, 5 tables
The chapter contains pyrolysis classification and characteristics, technologies of slow pyrolysis as the most efficient method of turning biomass into biochar, torrefaction - the mild pyrolysis, its mechanism, temperature regimes, production of torrefied pellets, fast pyrolysis -process of liquid fuel production from lignocellulosic biomass, composition and properties of bio-oil, flash and ultra-rapid pyrolysis (flash hydro-pyrolysis, rapid thermal process, solar flash pyrolysis, vacuum flash pyrolysis), solar driven pyrolysis, advantages, experimental study, modern pyrolyzer types (fixed or moving bed reactors, fluidized bed reactors, circulating fluidized bed, ultra-rapid, ablative, rotating-cone, vacuum pyrolyzers.
Chapter 2: Gasification (pp 31-38), 5 figures, 2 tables
The chapter contains potential paths for gasification of biomass, gasification reactions, steam and air gasification, types of gasifiers, updraft and downdraft fixed or moving bed gasifiers, fluidized bed gasifiers, dual fluidized bed gasification technology
Chapter 3: Poly-generation of heat, power and biofuel (pp.38-40), 2 figures
The chapter contains the technology of poly-generation of heat and biofuel from biomass.
1.6.1.Introduction
The major challenges for further mankind development is to achieve economic growth, supply sufficient food and energy resources for growing population, while preserving the environment [1, 2]. To meet these challenges, we should radically reduce consuming finite reserves of fossil fuels (coal, oil, natural gas), the use of which contributes to global warming and environment polluting. Instead, the world should move towards renewable energy sources such as wind energy, solar energy and biomass. At the same time, energy should be produced and consumed more efficiently. Energy conversion technologies need to be optimized to meet demands for lower costs, increased fuel flexibility, lower emissions and increased efficiency, for fossil fuels as well as renewable fuels.
Renewable energy share of gross final consumption of energy in the EU is targeted to increase from 8.5% in 2005 to 20% in 2020 [3]. Biomass is the dominant renewable energy source with a share of primary renewable energy production of 69% in 2008 (wood – 47%, municipal solid waste – 10%, biofuel – 6.9%, biogas – 5.1%). Wider use of biomass, a clean, renewable and CO2 neutral feedstock may extend the lifetime of our fossil fuels resources, for example by means of co-conversion, and alleviate global warming problems.
Bioenergy is the term used for energy associated to biomass, and biofuel is the bioenergy carrier, transporting solar energy stored as chemical energy. Biofuels can be considered as a renewable source of energy as long as they are based on sustainable biomass production. Biomass includes all kinds of materials that were directly or indirectly derived not too long ago from contemporary photosynthesis reactions, such as vegetal matter and its derivatives: wood fuel, wood-derived fuels, fuel crops, agricultural and agro-industrial by-products, and animal by-products, sewage sludge, etc. [2, 4].
Biomass resource can be subdivided into three categories [5]:
Forest products: Wood, logging residues, trees, shrubs and wood residues, sawdust, bark, etc., from forest clearings.
Wastes: Agricultural production wastes, agricultural processing wastes, crop residues, mill wood wastes, urban wood-wastes, urban organic wastes.
Energy crops: Short rotation woody crops, herbaceous woody crops, grasses, starch crops (corn, wheat and barley), sugar crops (cane and beet), oilseed crops (soyabean, sunflower, safflower).
Biomass is not a well-defined and often inhomogeneous feedstock, the composition of which may vary depending on age, origin, physical location, season and other factors. Biofuels differ in many aspects, such as their thermo-physical properties, calorific value, availability, cost, suitability as fuel for a power plant or conversion reactor. Utility of biomass as feedstock for conversion depends upon the chemical constituents and physical properties. Biomass contains varying amounts of cellulose, hemi-cellulose and lignin. Cellulose is generally the largest fraction (~ 40-50% in woods) followed by hemi-cellulose (~ 25-30%), lignin (~ 20-30%), ash (inorganic components, ~ 1.0%), extractives (minor aliphatic, aromatic compounds, alcohols, ketons and acids, esters, terpens), etc.
Cellulose is a polymer, consisting of linear chains of 1, 4-D-glucopyranose units, in which
the units are linked 1–4 in the alpha-configuration, with an average molecular weight of
around 100 000 kg/kmol. Hemi-celluloses are complex polysaccharides present in the cell wall, which consist of branched structures and vary with biomasses. It is a mixture of polysaccharides, composed almost entirely of sugars and methlyglucoronic and galaturonic acids, with an average molecular weight of 30 000 kg/kmol. Lignins are highly branched, substituted, mononuclear aromatic polymers in the cell walls of the certain biomass, especially woody species, and are often adjacent to cellulose fibers to form a ligno-cellulosic complex. Lignin is regarded as a group of amorphous, high molecular weight, chemically related compounds. The building blocks of lignin are believed to be a three-carbon chain attached to rings of six carbon atoms, called phenyl-propanes [5].
Heat and electricity are two forms of primary energy derived from biomass by means of direct combustion. Unlike fossil fuels, biomass cannot be handled, stored or transported easily especially in its use for transportation. This provides a major motivation for the conversion of solid biomass into liquid, gaseous and solid fuels, which can be achieved through one of two major paths - biochemical (fermentation) and thermochemical (pyrolysis, gasification), Figure 1.
Three types of primary fuel are produced from biomass [4]:
Solid (charcoal, torrefied biomass);
Gaseous (biogas (CH4, CO2), producer gas (CO, H2, CH4, CO2), syngas (CO, H2), substitute natural gas (CH4);
Liquid (ethanol, biodiesel, methanol, vegetable oil, and pyrolysis oil).
From the primary fuels come major categories of product:
Chemicals such as methanol, fertilizer, and synthetic fiber;
Energy (heat and electricity);
Transportation fuel such as gasoline and diesel.
Heat and electricity are two forms of primary energy derived from biomass. The use of biomass for efficient energy production is presently on the rise in developed countries because of its carbon-neutral feature.
Historically, the pyrolysis of biomass to produce charcoal was the first large-scale application of thermochemical conversion process. Such a technique which produces both charcoal (coke) and by-product gases (permanent and tars) is also called carbonization. Carbonized charcoal (biocarbon) is a renewable, highly reactive and calorific, CO2 neutral solid biofuel. Relative to fossil fuels, charcoal contains virtually no sulfur and very little nitrogen and ash. Carbonized charcoal can conduct electricity nearly as well as a metal, and it can have a high specific surface area. This extraordinary combination of properties causes charcoal to be the choice for such applications as ultra clean fuel for (co-) combustion (power production), cooking fuel (barbeque), sorbent (water treatment), metal reductant (production of silicon), biofuel for carbon fuel cell (power production), potting soil. Alternatively, there is now considerable interest in the use of biocarbons for soil beneficiation ("terra preta") and carbon sequestration [6, 7].
The use of ethanol and biodiesel as transport fuels reduces the emission of CO2 per unit of energy production. It also lessens dependence on fossil hydrocarbon fuel. Thus, biomass-based energy not only is renewable but is also clean from a greenhouse gas emission standpoint.
Bulky, moist and low calorific biomass cannot be handled, stored or transported easily, especially in its use for transportation. This provides a major motivation for the conversion of solid biomass into refined solid, liquid and gaseous fuels, which can be achieved through one of two major paths - biochemical (fermentation) and thermochemical (pyrolysis, gasification).
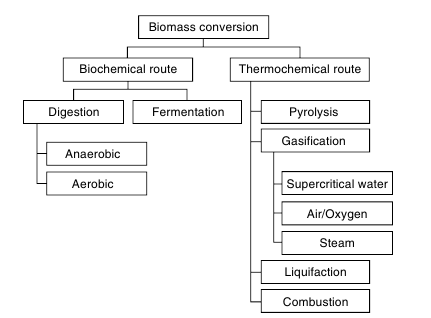
Figure 1. Biochemical and thermochemical paths of biomass conversion [4]
In the present chapter biochemical route of biomass conversion is not considered despite its practical importance concerning agricultural and agroindustrial biomass sources. An overview of thermochemical conversion technologies, products and potential end uses is shown in Figure 2. Choice of conversion process depends upon the type and quantity of biomass feedstock, the desired form of the energy (end use requirements), environmental standards, economic conditions and project specific factors. The following brief characterization of these processes is given in [4, 5].
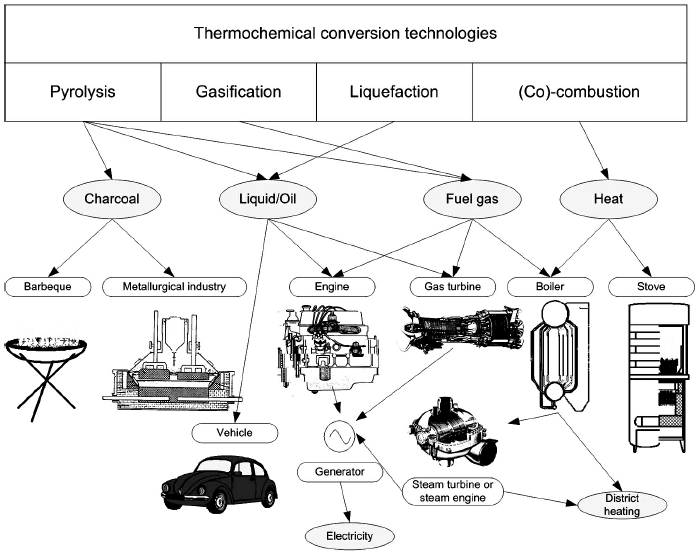
Figure 2. Thermochemical conversion technologies, products and potential end uses [2, 8]
Combustion. Biomass is directly burnt in the presence of oxidant media (air, oxygen, metal oxides) to convert chemical energy stored in biomass into heat, mechanical power, or electricity. In co-combustion (co-firing) processes biomass partly substitutes a (main) fossil fuel to synergistically enhance sustainability and environmental characteristics of the process.
Pyrolysis. Biomass is converted directly into solid, liquid and gaseous products by thermal decomposition in the absence of air/oxygen. Special techniques of pyrolysis admit the presence of such media as water steam, CO2 or hydrogen. Pyrolysis of biomass is carried out in the temperature range of 350–700 oC. Mild pyrolysis at temperatures below this range is called torrefacion.
Gasification. Biomass is converted into a combustible gas mixture by the partial oxidation of biomass at higher temperature in the range 800–900 oC.
Liquefaction. In this process, liquid is obtained by thermochemical conversion at low temperature and high pressure using a catalyst in the presence of hydrogen. It is an expensive process with the product being a tarry lump, which is difficult to handle.
Hydrogenation. This process is mainly for the production of methane by hydro-gasification, when first the syngas is formed and then CO is reacted with H2 to form methane.
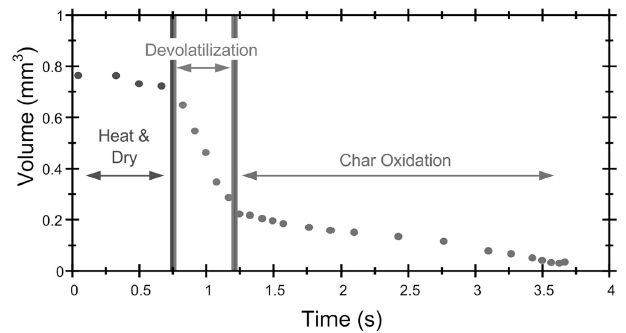
Figure 3. Heating, drying, pyrolysis (devolatilization) and char oxidation stages during combustion of a thermally small biomass particle
Being an independent conversion technique, pyrolysis occurs as an inevitable stage of biomass particle conversion during most the above thermochemical processes, following low-temperature stages of heating and drying. In Figure 3, showing temporal shrinking of a small burning particle, pyrolysis corresponds to the stage of devolatilization, i.e. release of gaseous pyrolysis products.
The present Chapter is focused on most promising methods of thermochemical conversion of biomass: pyrolysis, gasification and poly-generation of heat, power, biofuel.
