- •Van der Waals Equation
- •Thermodynamics
- •Internal Energy and the First Law of Thermodynamics
- •Infinitesimal Changes of State
- •19.7 Heat Capacities of an Ideal Gas
- •Restating the Second Law
- •The Carnot Cycle
- •The Carnot Refrigerator
- •Calculating Entropy: Microscopic States
- •Ideal Gases
- •1 Law of Thermodynamics
- •Internal Energy
Internal Energy and the First Law of Thermodynamics
Internal energy is one of the most important concepts in thermodynamics. Warming a body increases its internal energy and cooling the body decreases its internal energy. But what is internal energy? Matter consists of atoms and molecules, and these are made up of particles having kinetic and potential energies. We tentatively define the internal energy of a system as the sum of the kinetic energies of all of its constituent particles, plus the sum of all the potential energies of interaction among these particles.
CAUTION: is it internal? Note that internal energy does not include potential energy arising from the interaction between the system and its surroundings. If the system is a glass of water, placing it on a high shelf increases the gravitational potential energy arising from the interaction between the glass and the earth. But this has no effect on the interaction between the molecules of the water, and so the internal energy of the water does not change.
We
use the symbol
![]() for
internal energy. During a change of state of the system the internal
energy may change from an initial value
for
internal energy. During a change of state of the system the internal
energy may change from an initial value
![]() to
a final value
to
a final value
![]() .
We
denote the change in internal energy as
.
We
denote the change in internal energy as
![]() .
Experiments show that internal energy depends on number of degree of
freedom
.
Experiments show that internal energy depends on number of degree of
freedom
![]() ,
mass of an ideal gas, molar mass
and temperature
:
,
mass of an ideal gas, molar mass
and temperature
:
![]() .
(7)
.
(7)
Change
in internal energy of a given ideal gas depends only on change in
temperature
![]() :
:
![]() (8)
(8)
We
know that heat transfer is energy transfer. When we add a quantity of
heat
to
a system and the system does no work during the process, the internal
energy increases by an amount equal to
;
that
is,
![]() .
When
a system does work
by
expanding against its surroundings and no heat is added during the
process, energy
leaves the system and the internal energy decreases. That is, when
is
positive,
.
When
a system does work
by
expanding against its surroundings and no heat is added during the
process, energy
leaves the system and the internal energy decreases. That is, when
is
positive,
![]() is
negative, and vice versa. So
is
negative, and vice versa. So
![]() .
When both
heat
transfer and
work occur, the total
change
in internal energy is
.
When both
heat
transfer and
work occur, the total
change
in internal energy is
![]() (first
law of thermodynamics)
(9)
(first
law of thermodynamics)
(9)
We can rearrange this to the form
![]() (first
law of thermodynamics)
(10)
(first
law of thermodynamics)
(10)
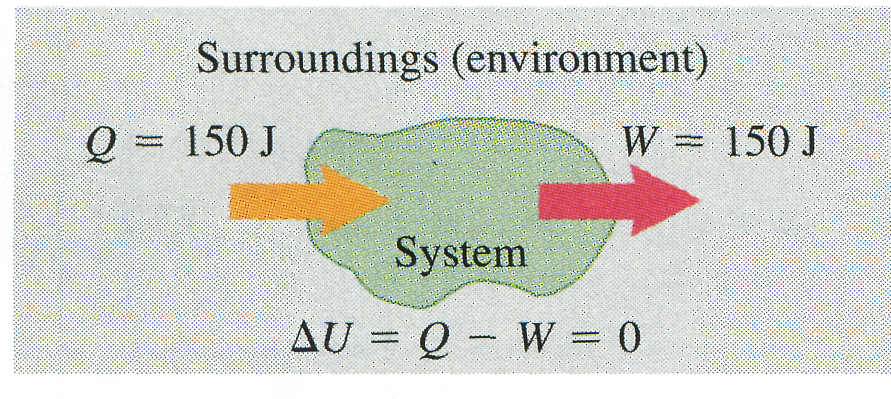
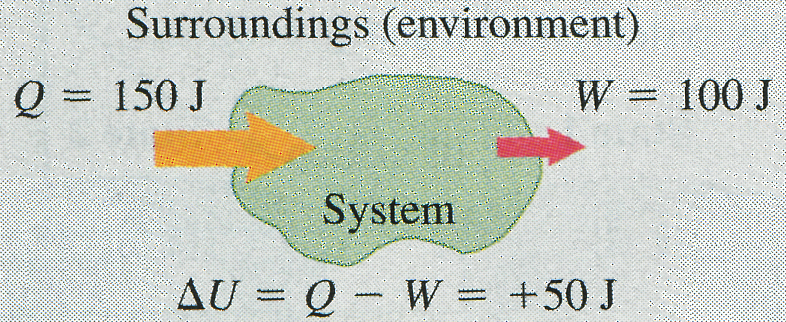
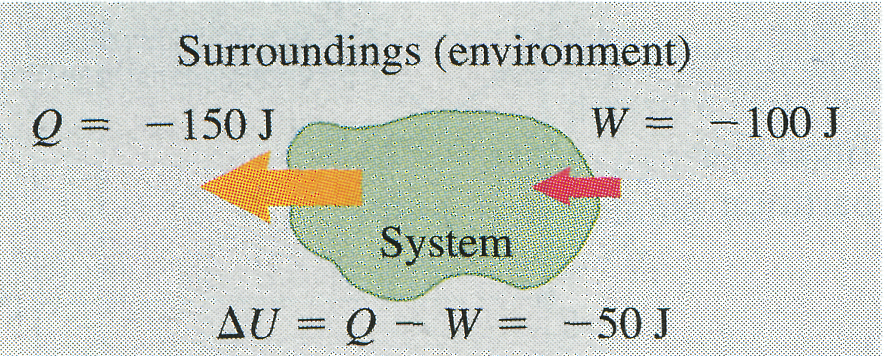
The message of Eq. (10) is that in general, when heat is added to a system, some of this added energy remains within the system, changing its internal energy by an amount ; the remainder leaves the system again as the system does work against its surroundings. Because and may be positive, negative, or zero, can be positive, negative, or zero for different processes (Fig. 8).
|
Fig.
8 In
a thermodynamic process, the internal
energy
of
a system may (a)
increase (![]() ),
(b) decrease (
),
(b) decrease (![]() ),
or (c) remain the same (
),
or (c) remain the same (![]() ).
).
(a) More heat is added to system than system does work: Internal energy of system increase; (b) More heat flows out of system than work is done: Internal energy of system decreases. (c) Heat added to system equals work done by system: Internal energy of system unchanged.
Equations (9) or (10) are the first law of thermodynamics. It is a generalization of the principle of conservation of energy to include energy transfer through heat as well as mechanical work.
For most systems other than ideal gases, the internal energy depends on pressure as well as temperature, so may vary even when is constant.