FINAL_TESTS_2010
.doc
25. Calculate pH of buffer solution, contained 0,1 mol CH3COOH and 1 mol CH3COONa in 1 L (pKacid =4,75).
Solution: _________________________________________________________________________
_________________________________________________________________________________
Answer:_________________ |
26. Calculate Redox potential of solution, contained 0,01 N К2CrO4 and 0,01 N CrCl3 per 1 L at рН=3 (Eo = + 1,33 V).
Solution: _________________________________________________________________________
_________________________________________________________________________________
Answer:_________________ |
27. Calculate dissociation degree α of 0,01 N NH4OH (K dis=1,7610-5)
Solution:___________________________________________________
Answer:__ α =______
28. Equivalent mass of Cu2+ (A=63,55 g/mol) cation for iodometric determination is:
1 |
63,55 g-eq/g; |
Answer:_____________ |
2 |
31,78 g-eq/g; |
|
3 |
16,71 g-eq/g; |
|
4 |
13,75 g-eq/g; |
|
5 |
125,10 g-eq/g. |
29. Note ionic and coordinative bonds in the complex of EDTA and metal ion:
|
C H2 |
|
CH2 |
-COONa |
|
|
N
|
|
|
NaCOO- |
C H2 |
|
CH2 |
-
|





 Me
Me
30. Calculate concentration of solution, contained 0,006 g of solute in 100 g of solution.
1 |
6 ppm; |
Answer:_____________ |
2 |
0,6 ppm; |
|
3 |
60 ppm; |
|
4 |
600 ppm; |
|
5 |
0,006 ppm. |
НАЦІОНАЛЬНИЙ УНІВЕРСИТЕТ БІОРЕСУРСІВ ТА ПРИРОДОКОРИСТУВАННЯ УКРАЇНИ
Факультет Екології і біотехнології
Напрям підготовки (спеціальність) 0514 – Біотехнологія
(6.051401 – Екобіотехнологія)
Форма навчання денна (англійською мовою)Семестр 3 Курс 2
ОКР «Бакалавр»
Кафедра аналітичної і біонеорганічної хімії та якості води
Дисципліна Аналітична хімія
Викладач _____ ( Войтенко Л.В. )
«Затверджую»
Завідувач кафедри ( Косматий В.Є. )
«09» грудня 2009 р.Студент 2 курсу 2 групи факультету екології і біотехнології
(прізвище, ім’я, по-батькові) (підпис)
Дата проведення іспиту – „03” лютого 2010 року.
Білет № 4
1. Put in the sentence a missing words:
Analytical chemistry includes two parts: _________________________ and __________________ analyses.
|
2. Note specific qualitative reagent of Mn2+ cations is:
1 |
Sodium Bismuthate NaBiO3 in nitrate acid medium |
Answer:____________________ |
2 |
Yellow blood salt K4[Fe(CN)6] |
|
3 |
Red blood salt K3[Fe(CN)6] |
|
4 |
Dimethylglioxime (Chugaev’s reagent) C4H8N2O2 |
3. To point the correspondence of the compound formulas and type of the analytical reagents (ammonium-phosphate classification): (possible more than one true variant)
A. B. C.
|
Specific Selective Group
|
1 2
3 4 5 6 |
(NH4)2HPO4 Potassium hexanitritocobaltate (III) Na3[Co(NO2)6] H2SO4 Nessler’s reagent K2[HgI4]+KOH NaBiO3 + HNO3 (diluted) HCl |
Answer: А - ________; С - ________;
В -_________; D - ________. |
4. To point the correspondence of names of analytical glassware:
A. B. C. D.
|
Electrical balance Dessicator Convection drier Centrifuge |
1 2 3 4 |
Answer: А - ____; В -____; С - ____; D - ____, E -____.
5. Calculate equivalent mass of K2CrO4 (M=194,20 g/mol) for RedOx titration in acidic medium:
1 |
194,20 g/g-eq; |
Answer:____________________ |
2 |
97,10 g/g-eq; |
|
3 |
64,73 g/g-eq; |
|
4 |
32,37 g/g-eq. |
6. To propose the reagent for the separation of cations:
|
K+, Na+
↓
Added
Reactant____________________ |
|
Solution:_______ Cation |
|
Sediment:_______ (chemical formula) |
7. Determine correspondence of titration methods and their secondary standards (working solutions).
A B C D |
Neutralization Complexonometry Iodometry Permanganatometry |
1 2 3 4 |
Na2S2O3 (Sodium Thyosulfate) HCl (Hydrochloric acid) Na2EDTA (trilon B) KMnO4 (potassium permanganate) |
Answer: А - _______; В - _______; С - _______; D - _______. |
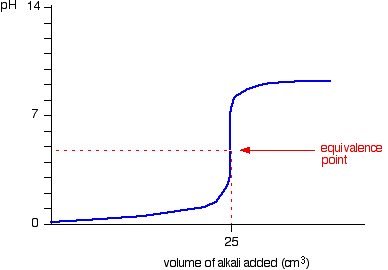
8. This figure demonstrates the curve of titration of _______ (weak or strong) _________ (acid or base) by _________ (weak or strong)____________ (acid or base).
9. Calculate ionic power µ (or I) of solution, contained 0,02 M CaCl2 in 1 L.
1. |
0,05 |
Answer:____________________ |
2. |
0,03 |
|
3. |
0,06 |
|
4. |
0,09 |
10. Note relative specific reagent of Fe2+ cation
1 |
Sodium Bismuthate NaBiO3Zinc-Uranyl-Acetate |
Answer:___________________ |
2 |
Potassium ferrocyanide K3[Fe(CN)6] |
|
3 |
Potassium ferricyanide K4 [Fe(CN)6] |
|
4 |
Ammonium Hydrophosphate (NH4)2HPO4. |
11. To propose the buffer mixture, using proposed reactants (possible more than one true variant)
1 |
HCl, CH3COOH |
Answer:_______________________________
_______________________________ |
2 |
NH4OH, LiOH |
|
3 |
NH4Cl, LiCl, CH3COONa |
12. ppm concentration is shown:
1 |
Quantity of soluble substance in equivalents per 1 000 L of solution; |
Answer:______ |
2 |
Quantity of soluble substance in grams per 1 000 grams of solution; |
|
3 |
Quantity of soluble substance in grams per 1 000 000 grams of solution; |
|
4 |
Quantity of soluble substance in mol per 100 g of solution. |
13. Note the most sensitive reactions for the next cations:
A. B. C.
|
NH4+ Na+ K+ |
1. 2. 3. 4. 5. 6. |
Zinc-Uranyl-Acetate Zn(UO2)3(CH3COO)8 Nessler’s reagent K2[HgI4]+KOH Sodium hydrotartrate NaHC4H4O6 Potassium hexanitritocobaltate (III) Na3[Co(NO2)6] Alkalis at heating Potassium hexahydroxostibiate K[Sb(OH)6] |
Answer: А - ______;
В - ______;
С - ______. |
14. To note heterogeneous systems, formed at mixing of solutions by pairs
KOH; HCl; H3PO4; Al(OH)3
Answer:________- _____.
15. To write reaction of Ni2+ Chloride and Dimethylglioxime (Chugaev’s reagent) C4H8N2O2 in ammonia medium (pH=9) (in molecular form) excess of ammonia thiocyanate in molecular form (coordinative number of Co3+ is 6) and to calculate the sum of coefficients in this reaction (remember, that absence of coefficient before formula means figure 1):
__________ + ___ ____________ → _____________________ + __ ______________
Sum of coefficients: ______
16. It is necessary for preparation of amphoteric sediment:
1 |
To precipitate of hot concentrated solutions; |
Answer:____________
|
2 |
To precipitate of cold diluted solutions; |
|
3 |
To precipitate of hot diluted solutions; |
|
4 |
To precipitate of cold concentrated solutions. |
17. Red-Ox potential of system CrO42-/Cr3+ in acidic medium at pH=1 and concentrations of [CrO42-]=0,1 M; [Cr3+]=0,01 M is (E0 = +1,36 V):
1 |
+ 1,456 V; |
Answer:____________
|
2 |
+ 1,222 V; |
|
3 |
+ 1,486 V; |
|
4 |
+ 1,782 V. |
18. Determine mass of MgSO4·7H2O (M=246,47 g/mol) for gravimetric determination of Magnesium content in the form of sediment MgNH4PO4 (M=137,35 g/mol):
Solution: _________________________________________________________________________
_________________________________________________________________________________
Answer:_________________
19. Note the name of this phenomenon
← ?
Answer:_________________
20. Calculate solubility (in mol/L and g/L) of SrCO3 (SP= 5,60·10-10, M(SrCO3)= 147,63 g/mol) in the solution of ionic power 0,05 M (activity coefficients – see Appendix).
Solution:_________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________ Answer:_________ |
21. What sediment is the best choice for gravimetric determination of zinc (II) :
1 |
ZnSe (SP=1·10-31) |
Answer:____________
|
2 |
ZnCO3 (SP=1,45·10-11) |
|
3 |
ZnS (SP=2·10-25) |
|
4 |
ZnSO3(SP=1,2·10-17) |
22. Determine note of molarity (M):
Answer: Molarity is_______________________________________________________________
_________________________________________________________________________________
23. Calculate pH of 0,01 N (NH4)2CO3 (pK1 (H2CO3) =6,35; pK(NH4OH) =4,75).
Solution: _________________________________________________________________________
_________________________________________________________________________________
Answer: ___________
24. Calculate titr T of 15% H2SO4 solution (density d=1,102 g/cm3, M=98,00 g/mol):
1 |
6,0497 g/mL; |
Answer:_____________ |
2 |
0,3490 g/mL; |
|
3 |
0,1653 g/mL; |
|
4 |
0,1790 g/mL. |
25. Calculate pH of buffer solution, contained 1 mol CH3COOH and 0,01 mol CH3COONa in 1 L (pKacid =4,75).
Solution: _________________________________________________________________________
_________________________________________________________________________________
Answer:_________________ |
26. Calculate Redox potential of solution, contained 0,1 N К2CrO4 and 0,1 N CrCl3 per 1 L at рН=1 (Eo = + 1,33 V).
Solution: _________________________________________________________________________
_________________________________________________________________________________
Answer:_________________ |
27. Indicators of complexonometry are:
1 |
Weak organic bases and bases; |
Answer:_____________ |
2 |
Strong organic acids; |
|
3 |
Red-Ox systems; |
|
4 |
Weak ligands, formed color compexes with metal cations. |
28. Equivalent mass of H3PO4 (A=98,00 g/mol) for neutralization method:
1 |
98,00 g-eq/g; |
Answer:_____________ |
2 |
49,00 g-eq/g; |
|
3 |
32,67 g-eq/g; |
|
4 |
13,75 g-eq/g; |
|
5 |
190,22 g-eq/g. |
29. Put in the sentence a missing words:
______________ indicators are oxidation-reducing pairs, where oxidized and reduced forms differ
in color. |
30. Determine the correspondence between titration system and the best indicator:
|
Titration system |
|
Indicator |
Answer:
A-____________;
B-____________;
C-____________. |
A.
B.
C.
|
0,1 N hydrochloric acid HCl by 0,1 N Sodium hydroxide NaOH (pHeq=7,00) 0,1 N acetic acid CH3COOH by 0,1 N Sodium hydroxide NaOH (pHeq=8,87) 0,1 N hydrochloric acid HCl by 0,1 N Ammonium hydroxide NH4OH (pHeq=5,73) |
1 2 3 4 5 6 7 8 |
Alizarin yellow R (pT=11,3) Thymol Blue (pT=1,7) Phenol red (pT=5,8) Bromophenol blue (pT=4,1) Litmus neutral (pT= 7,1) Thymol blue (pT=8,9) Cresol red (pT=8,2) Phenolphthalein (pT=9,6)
|
НАЦІОНАЛЬНИЙ УНІВЕРСИТЕТ БІОРЕСУРСІВ ТА ПРИРОДОКОРИСТУВАННЯ УКРАЇНИ
Факультет Екології і біотехнології
Напрям підготовки (спеціальність) 0514 – Біотехнологія
(6.051401 – Екобіотехнологія)
Форма навчання денна (англійською мовою)
Семестр 3 Курс 2
ОКР «Бакалавр»
Кафедра аналітичної і біонеорганічної хімії та якості води
Дисципліна Аналітична хімія
Викладач _____ ( Войтенко Л.В. )
«Затверджую»
Завідувач кафедри ( Косматий В.Є. )
«09» грудня 2009 р.
Студент 2 курсу 2 групи факультету екології і біотехнології
(прізвище, ім’я, по-батькові) (підпис)
Дата проведення іспиту – „03” лютого 2010 року.
Білет № 5
1. Put in the sentence a missing words:
Detectible minimum (m) is the _____________ quantity of substance or ion, which can be detected by given reaction at certain conditions. |
2. Note specific qualitative reagent of Co2+ cations:
1 |
NH4SCN in acetone |
Answer:______________________ |
2 |
Yellow blood salt K4[Fe(CN)6] |
|
3 |
Red blood salt K3[Fe(CN)6] |
|
4 |
Dimethylglioxime (Chugaev’s reagent) C4H8N2O2 |
3. To point the correspondence of the compound formulas and type of the analytical reagents (ammonium-phosphate classification): (possible more than one true variant)
A. B. C.
|
Specific Selective Group
|
1 2 3 4 5 6 |
(NH4)2HPO4 Potassium hexanitritocobaltate (III) Na3[Co(NO2)6] Nessler’s reagent K2[HgI4]+KOH H2SO4 Sodium hydrotartrate NaHC4H4O6 HCl |
Answer: А - ________;
В -_________;
С - ________. |
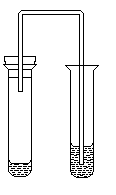
4. Process accompanied rapid precipitating and in resulting contaminated a sediment (see figure), is named (answer as one word)_____________________.
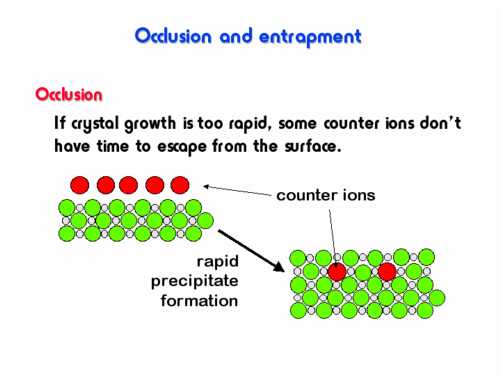
5. Calculate equivalent mass of KMnO4 (M=158 g/mol) for RedOx titration in acidic medium:
1 |
31,6 g/g-eq |
Answer:____________________ |
2 |
79,0 g/g-eq |
|
3 |
52,7 g/g-eq |
|
4 |
158 g/g-eq |
6. To propose the reagent for the separation of cations:
|
Mg2+, Pb2+ ↓ |
Added
Reactant____________________ |
Solution:_______ Cation |
|
Sediment:_______ (chemical formula) |
7. Determine correspondence of titration methods and their indicators:
A B C D |
Neutralization Complexonometry Iodometry Permanganatometry |
1 2 3 4 |
Diphenylammine Litmus Starch Eriochrome black T |
Answer: А - _______; В - _______; С - _______; D - _______. |
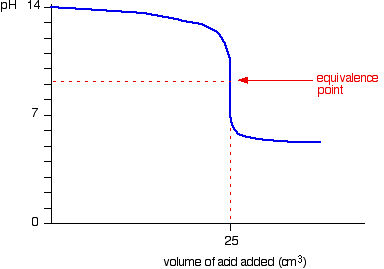
8. This figure demonstrates the curve of titration of _______ (weak or strong) _________ (acid or base) by _________ (weak or strong)____________ (acid or base).
9. Calculate ionic power µ (or I) of solution, contained 0,1 M K2SO3 in 1 L.
1. |
0,50 |
Answer:____________________ |
2. |
0,30 |
|
3. |
0,10 |
|
4. |
0,09 |
10. Note relative specific reagent of Fe3+ cation
1 |
Sodium Bismuthate NaBiO3Zinc-Uranyl-Acetate |
Answer:___________________ |
2 |
Potassium ferrocyanide K3[Fe(CN)6] |
|
3 |
Potassium ferricyanide K4 [Fe(CN)6] |
|
4 |
Ammonium Hydrophosphate (NH4)2HPO4. |
11. To propose the buffer mixture, using proposed reactants (possible more than one true variant)
1 |
NH2Cl, Na2HPO4; NaH2PO4 |
Answer:_____________________________________________________________________________ |
2 |
NH2OH; KOH |
|
3 |
HCl, H3PO4 |
12. Titr concentration is shown:
1 |
Quantity of soluble substance in grams per 1 L of solution; |
Answer:______ |
2 |
Quantity of soluble substance in grams per 1 grams of solution; |
|
3 |
Quantity of soluble substance in grams per 1 mL of solution; |
|
4 |
Quantity of soluble substance in mol per 100 mL of solution. |
13. Apparatus, presented at picture, is used for identification of anions:
|
Answer:___________________ |


 OOC -
OOC - -CH2-CH2-N
-CH2-CH2-N
 COO
COO