
Borchers Andrea Ann (ed.) Handbook of Signs & Symptoms 2015
.pdf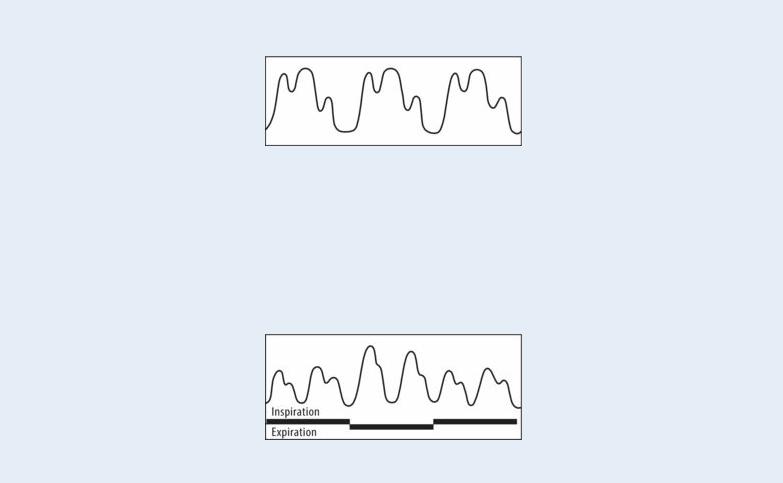
high cardiac output states.
PULSUS PARADOXUS
Pulsus paradoxus is an exaggerated decline in blood pressure during inspiration, resulting from an increase in negative intrathoracic pressure. Pulsus paradoxus that exceeds 10 mm Hg is considered abnormal and may result from cardiac tamponade, constrictive pericarditis, or severe lung disease.
Special Considerations
Prepare the patient for diagnostic tests, such as an electrocardiogram, chest X-ray, cardiac catheterization, or angiography, to help determine the underlying cause of the abnormal pulse.
Patient Counseling
Explain which signs and symptoms of heart failure to report and the importance of planning rest periods.
Pediatric Pointers
Pulsus bisferiens may be palpated in children with a large patent ductus arteriosus as well as those with congenital aortic stenosis and insufficiency.
Pulsus Paradoxus
Pulsus paradoxus, or paradoxical pulse, is an exaggerated decline in blood pressure during inspiration. Normally, systolic pressure falls less than 10 mm Hg during inspiration. In pulsus paradoxus, it falls more than 10 mm Hg. (See Comparing Arterial Pressure Waves , page 608.) When systolic pressure falls more than 20 mm Hg, the peripheral pulses may be barely palpable or may disappear during inspiration.
Pulsus paradoxus is thought to result from an exaggerated inspirational increase in negative intrathoracic pressure. Normally, systolic pressure drops during inspiration because of blood pooling

in the pulmonary system. This, in turn, reduces left ventricular filling and stroke volume and transmits negative intrathoracic pressure to the aorta. Conditions associated with large intrapleural pressure swings, such as asthma, or those that reduce left-sided heart filling, such as pericardial tamponade, produce pulsus paradoxus.
To accurately detect and measure pulsus paradoxus, use a sphygmomanometer or an intra-arterial monitoring device. Inflate the blood pressure cuff 10 to 20 mm Hg beyond the peak systolic pressure. Then, deflate the cuff at a rate of 2 mm Hg/second until you hear the first Korotkoff sound during expiration. Note the systolic pressure. As you continue to slowly deflate the cuff, observe the patient’s respiratory pattern. If pulsus paradoxus is present, the Korotkoff sounds will disappear with inspiration and return with expiration. Continue to deflate the cuff until you hear Korotkoff sounds during inspiration and expiration, and, again, note the systolic pressure. Subtract this reading from the first one to determine the degree of pulsus paradoxus. A difference of more than 10 mm Hg is abnormal.
You can also detect pulsus paradoxus by palpating the radial pulse over several cycles of slow inspiration and expiration. Marked pulse diminution during inspiration indicates pulsus paradoxus. When you check for pulsus paradoxus, remember that irregular heart rhythms and tachycardia cause variations in pulse amplitude and must be ruled out before true pulsus paradoxus can be identified.
 EMERGENCY INTERVENTIONS
EMERGENCY INTERVENTIONS
Pulsus paradoxus may signal cardiac tamponade — a life-threatening complication of pericardial effusion that occurs when sufficient blood or fluid accumulates to compress the heart. When you detect pulsus paradoxus, quickly take the patient’s other vital signs. Check for additional signs and symptoms of cardiac tamponade, such as dyspnea, tachypnea, diaphoresis, jugular vein distention, tachycardia, narrowed pulse pressure, and hypotension. Emergency pericardiocentesis to aspirate blood or fluid from the pericardial sac may be necessary. Then, evaluate the effectiveness of pericardiocentesis by measuring the degree of pulsus paradoxus; it should decrease after aspiration.
History and Physical Examination
If the patient doesn’t have cardiac tamponade, find out if he has a history of chronic cardiac or pulmonary disease. Ask about the development of associated signs and symptoms, such as a cough or chest pain. Then, auscultate for abnormal breath sounds.
Medical Causes
Cardiac tamponade. Pulsus paradoxus commonly occurs with cardiac tamponade, but it may be difficult to detect if intrapericardial pressure rises abruptly and profound hypotension occurs. With severe tamponade, assessment also reveals these classic findings: hypotension, diminished or muffled heart sounds, and jugular vein distention. Related findings include chest pain, a pericardial friction rub, narrowed pulse pressure, anxiety, restlessness, clammy skin, and hepatomegaly. Characteristic respiratory signs and symptoms include dyspnea, tachypnea, and cyanosis; the patient typically sits up and leans forward to facilitate breathing.
If cardiac tamponade develops gradually, pulsus paradoxus may be accompanied by weakness,

anorexia, and weight loss. The patient may also report chest pain, but he won’t have muffled heart sounds or severe hypotension.
Chronic obstructive pulmonary disease (COPD). The wide fluctuations in intrathoracic pressure that characterize COPD produce pulsus paradoxus and possibly tachycardia. Other findings vary, but may include dyspnea, tachypnea, wheezing, a productive or nonproductive cough, accessory muscle use, barrel chest, and clubbing. The patient may show labored, pursedlip breathing after exertion or even at rest. He typically sits up and leans forward to facilitate breathing. Auscultation reveals decreased breath sounds, rhonchi, and crackles. Weight loss, cyanosis, and edema may occur.
Pericarditis (chronic constrictive). Pulsus paradoxus can occur in up to 50% of patients with pericarditis. Other findings include a pericardial friction rub, chest pain, exertional dyspnea, orthopnea, hepatomegaly, and ascites. Patients also exhibit peripheral edema and Kussmaul’s sign — jugular vein distention that becomes more prominent on inspiration.
Pulmonary embolism (massive). Decreased left ventricular filling and stroke volume in massive pulmonary embolism produce pulsus paradoxus as well as syncope and severe apprehension, dyspnea, tachypnea, and pleuritic chest pain. The patient appears cyanotic, with jugular vein distention. He may succumb to circulatory collapse, with hypotension and a weak, rapid pulse. Pulmonary infarction may produce hemoptysis along with decreased breath sounds and a pleural friction rub over the affected area.
Special Considerations
Prepare the patient for an echocardiogram to visualize cardiac motion and to help determine the causative disorder. Also, monitor his vital signs, and frequently check the degree of paradox. An increase in the degree of paradox may indicate recurring or worsening cardiac tamponade or impending respiratory arrest in severe COPD. Vigorous respiratory treatment, such as chest physiotherapy, may avert the need for endotracheal intubation.
Patient Counseling
Explain self-care techniques to the patient with COPD (pursed-lip diaphragmatic breathing, coughing, and deep breathing exercises) as well as the proper use of home oxygen equipment. Emphasize the importance of prescribed medications and their adverse effects.
Pediatric Pointers
Pulsus paradoxus commonly occurs in children with chronic pulmonary disease, especially during an acute asthma attack. Children with pericarditis may also develop pulsus paradoxus due to cardiac tamponade, although this disorder more commonly affects adults. Pulsus paradoxus above 20 mm Hg is a reliable indicator of cardiac tamponade in children; a change of 10 to 20 mm Hg is equivocal.
REFERENCES
Buttaro, T. M., Tybulski, J., Bailey, P. P. , & Sandberg-Cook, J. (2008) . Primary care: A collaborative practice (pp. 444–447) . St. Louis, MO: Mosby Elsevier.
McCance, K. L., Huether, S. E., Brashers, V. L. , & Rote, N. S. (2010). Pathophysiology: The biologic basis for disease in adults and children. Maryland Heights, MO: Mosby Elsevier.
Sommers, M. S., & Brunner, L. S. (2012). Pocket diseases. Philadelphia, PA: F.A. Davis.

Pupils, Nonreactive
Nonreactive (fixed) pupils fail to constrict in response to light or to dilate when the light is removed. The development of a unilateral or bilateral nonreactive response indicates an important change in the patient’s condition and may signal a life-threatening emergency and possibly brain death. It also occurs with the use of certain ophthalmic drugs.
To evaluate pupillary reaction to light, first test the patient’s direct light reflex. Darken the room, and cover one of the patient’s eyes while you hold open the opposite eyelid. Using a bright penlight, bring the light toward the patient from the side and shine it directly into his opened eye. If normal, the pupil will promptly constrict. Next, test the consensual light reflex. Hold the patient’s eyelids open, and shine the light into one eye while watching the pupil of the opposite eye. If normal, both pupils will promptly constrict. Repeat both procedures in the opposite eye. A unilateral or bilateral nonreactive response indicates dysfunction of cranial nerves (CNs) II and III, which mediate the pupillary light reflex. (See Innervation of Direct and Consensual Light Reflexes, page 614.)
 EMERGENCY INTERVENTIONS
EMERGENCY INTERVENTIONS
If the patient is unconscious and develops unilateral or bilateral nonreactive pupils, quickly take his vital signs. Be alert for decerebrate or decorticate posture, bradycardia, elevated systolic blood pressure, widened pulse pressure, and the development of other untoward changes in the patient’s condition. Remember, a unilateral dilated, nonreactive pupil may be an early sign of uncal brain herniation. Emergency surgery to decrease intracranial pressure (ICP) may be necessary. If the patient isn’t already being treated for increased ICP, insert an I.V. line to administer a diuretic, an osmotic, or a corticosteroid. You may also need to start the patient on controlled hyperventilation.
History and Physical Examination
If the patient is conscious, obtain a brief history. Ask him what type of eye drops he’s using, if any, and when they were last instilled. Also, ask if he’s experiencing pain and, if so, try to determine its location, intensity, and duration. Check the patient’s visual acuity in both eyes. Then, test the pupillary reaction to accommodation: Normally, both pupils constrict equally as the patient shifts his glance from a distant to a near object.
Next, hold a penlight at the side of each eye, and examine the cornea and iris for abnormalities. Measure intraocular pressure (IOP) with a tonometer, or estimate IOP by placing your second and third fingers over the patient’s closed eyelid. If the eyeball feels rock-hard, suspect elevated IOP. Ophthalmoscopic and slit-lamp examinations of the eye will need to be performed. If the patient has experienced ocular trauma, don’t manipulate the affected eye. After the examination, make sure to cover the affected eye with a protective metal shield, but don’t let the shield rest on the globe.
 EXAMINATION TIP Innervation of Direct and Consensual
EXAMINATION TIP Innervation of Direct and Consensual
Light Reflexes
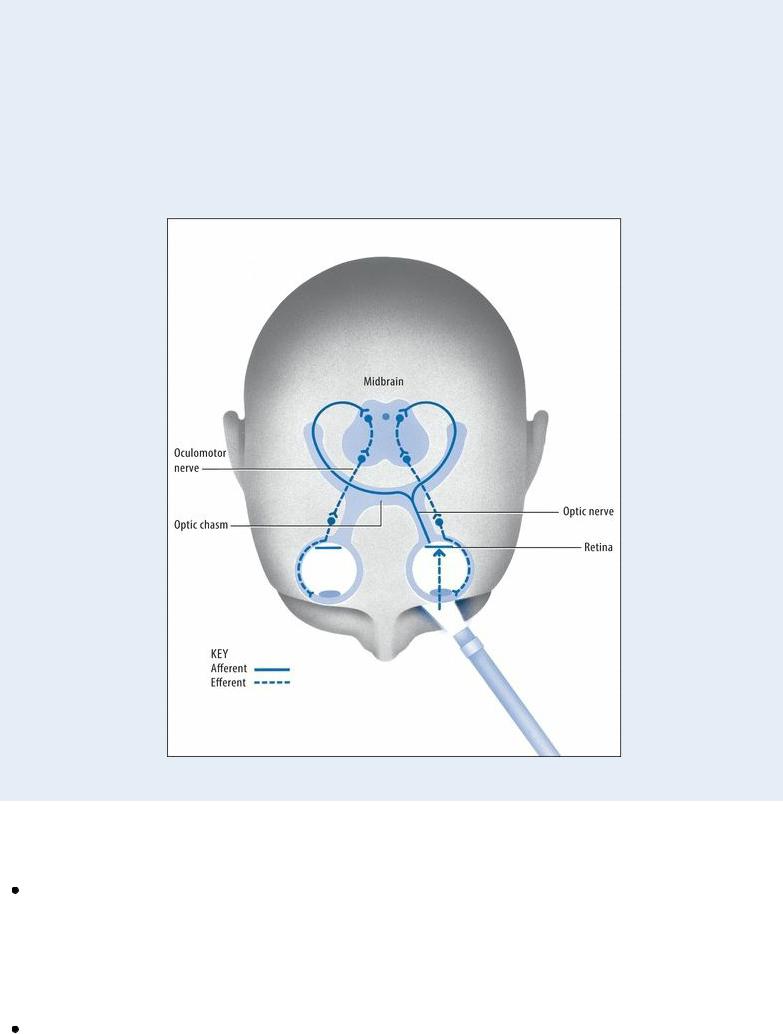
Two reactions — direct and consensual — constitute the pupillary light reflex. Normally, when a light is shined directly onto the retina of one eye, the parasympathetic nerves are stimulated to cause brisk constriction of that pupil — the direct light reflex . The pupil of the opposite eye also constricts — the consensual light reflex.
The optic nerve (cranial nerve [CN] II) mediates the afferent arc of this reflex from each eye, whereas the oculomotor nerve (CN III) mediates the efferent arc to both eyes. A nonreactive or sluggish response in one or both pupils indicates dysfunction of these cranial nerves, usually due to degenerative disease of the central nervous system.
Medical Causes
Botulism. Bilateral mydriasis and nonreactive pupils usually appear 12 to 36 hours after eating tainted food. Other early findings include blurred vision, diplopia, ptosis, strabismus, and extraocular muscle palsies, along with anorexia, nausea, vomiting, diarrhea, and dry mouth. Vertigo, deafness, hoarseness, a nasal voice, dysarthria, and dysphagia follow. Progressive muscle weakness and absent deep tendon reflexes usually evolve over 2 to 4 days, resulting in severe constipation and paralysis of respiratory muscles with respiratory distress.
Encephalitis. As encephalitis progresses, initially sluggish pupils become dilated and

nonreactive. Decreased accommodation and other symptoms of cranial nerve palsies, such as dysphagia, develop. Within 48 hours after onset, encephalitis causes a decreased level of consciousness, a high fever, a headache, vomiting, and nuchal rigidity. Aphasia, ataxia, nystagmus, hemiparesis, and photophobia may occur with seizures.
Glaucoma (acute angle closure). With acute angle-closure glaucoma, an ophthalmic emergency, examination reveals a moderately dilated, nonreactive pupil in the affected eye. Conjunctival injection, corneal clouding, and decreased visual acuity also occur. The patient experiences a sudden onset of blurred vision, followed by excruciating pain in and around the affected eye. He commonly reports seeing halos around white lights at night. Severely elevated IOP commonly induces nausea and vomiting.
Oculomotor nerve palsy. Commonly, the first signs of oculomotor nerve palsy are a dilated, nonreactive pupil and loss of the accommodation reaction. These findings may occur in one or both eyes, depending on whether the palsy is unilateral or bilateral. Among the causes of total CN III palsy is life-threatening brain herniation. Central herniation causes bilateral midposition nonreactive pupils, whereas uncal herniation initially causes a unilateral dilated, nonreactive pupil. Other common findings include diplopia, ptosis, outward deviation of the eye, and an inability to elevate or adduct the eye. Additional findings depend on the underlying cause of the palsy.
Uveitis. A small, nonreactive pupil that appears suddenly with severe eye pain, conjunctival injection, and photophobia typifies anterior uveitis. With posterior uveitis, similar features develop insidiously, along with blurred vision and a distorted pupil shape.
Other Causes
Drugs. Instillation of a topical mydriatic and a cycloplegic may induce a temporarily nonreactive pupil in the affected eye. Opiates, such as heroin and morphine, cause pinpoint pupils with a minimal light response that can be seen only with a magnifying glass. Atropine poisoning produces widely dilated, nonreactive pupils.
Special Considerations
If the patient is conscious, monitor his pupillary light reflex to detect changes. If he’s unconscious, close his eyes to prevent corneal exposure. (Use tape to secure the eyelids, if needed.)
Patient Counseling
Teach proper methods for instilling eye drops, and explain ways to reduce photophobia. Stress the importance of follow-up care to check IOP.
Pediatric Pointers
Children have nonreactive pupils for the same reasons as adults. The most common cause is oculomotor nerve palsy from increased ICP.
REFERENCES
Biswas, J. , Krishnakumar, S., & Ahuja, S. (2010) . Manual of ocular pathology. New Delhi, India: Jaypee—Highlights Medical

Publishers.
Eagle, R. C. Jr. (2011). Eye pathology: An atlas and text. Philadelphia, PA: Lippincott Williams & Wilkins. Gerstenblith, A. T., & Rabinowitz, M. P. (2012). The wills eye manual. Philadelphia, PA: Lippincott Williams & Wilkins. Roy, F. H. (2012). Ocular differential diagnosis. Clayton, Panama: Jaypee—Highlights Medical Publishers, Inc.
Pupils, Sluggish
A sluggish pupillary reaction is an abnormally slow pupillary response to light. It can occur in one pupil or both, unlike the normal reaction, which is always bilateral. A sluggish reaction accompanies degenerative disease of the central nervous system and diabetic neuropathy. It can occur normally in elderly people, whose pupils become smaller and less responsive with age.
To assess pupillary reaction to light, first test the patient’s direct light reflex. Darken the room, and cover one of the patient’s eyes while you hold open the opposite eyelid. Using a bright penlight, bring the light toward the patient from the side and shine it directly into his opened eye. If normal, the pupil will promptly constrict. Next, test the consensual light reflex. Hold both of the patient’s eyelids open, and shine the light into one eye while watching the pupil of the opposite eye. If normal, both pupils will promptly constrict. Repeat both procedures to test light reflexes in the opposite eye. A sluggish reaction in one or both pupils indicates dysfunction of cranial nerves II and III, which mediate the pupillary light reflex. (See Innervation of Direct and Consensual Light Reflexes, page 614.)
History and Physical Examination
If you detect a sluggish pupillary reaction, determine the patient’s visual function. Start by testing visual acuity in both eyes. Then, test the pupillary reaction to accommodation; the pupils should constrict equally as the patient shifts his glance from a distant to a near object.
Next, hold a penlight at the side of each eye, and examine the cornea and iris for irregularities, scars, and foreign bodies. Measure intraocular pressure (IOP) with a tonometer, or estimate IOP by placing your fingers over the patient’s closed eyelid. If the eyeball feels rock-hard, suspect elevated IOP. Also, ophthalmoscopic and slit-lamp examinations of the eye will need to be performed.
Medical Causes
Adie’s syndrome. Adie’s syndrome produces an abrupt onset of unilateral mydriasis and a sluggish pupillary response that may progress to a nonreactive response. The patient may complain of blurred vision and cramplike eye pain. Eventually, both eyes may be affected. Musculoskeletal assessment also reveals hypoactive or absent deep tendon reflexes in the arms and legs.
Encephalitis. Encephalitis initially produces a bilateral sluggish pupillary response. Later, pupils become dilated and nonreactive, and decreased accommodation may occur, along with other cranial nerve palsies, such as dysphagia and facial weakness. Within 24 to 48 hours after onset, encephalitis causes a decreased level of consciousness, a headache, a high fever, vomiting, and nuchal rigidity. Also, aphasia, ataxia, nystagmus, hemiparesis, and photophobia may occur. The patient may exhibit seizure activity and myoclonic jerks.
Herpes zoster. The patient with herpes zoster affecting the nasociliary nerve may have a sluggish pupillary response. Examination of the conjunctiva reveals follicles. Additional ocular

findings include a serous discharge, absence of tears, ptosis, and extraocular muscle palsy. Iritis (acute). With iritis, the affected eye exhibits a sluggish pupillary response and conjunctival injection. The pupil may remain constricted; if posterior synechiae have formed, the pupil will also be irregularly shaped. The patient reports a sudden onset of eye pain and photophobia and may also have blurred vision.
Myotonic dystrophy. With myotonic dystrophy, sluggish pupillary reaction may be accompanied by lid lag, ptosis, miosis and, possibly, diplopia. The patient may develop decreased visual acuity from cataract formation. Muscular weakness and atrophy and testicular atrophy may occur.
Tertiary syphilis. A sluggish pupillary reaction (especially in Argyll Robertson pupils) occurs in the late stage of neurosyphilis, along with marked weakness of the extraocular muscles, visual field defects and, possibly, cataractous changes in the lens. The patient may complain of orbital rim pain, which worsens at night. He may also exhibit lid edema, decreased visual acuity, and exophthalmos. Tertiary lesions appear on the skin and mucous membranes. Liver, respiratory, cardiovascular, and additional neurologic dysfunction may also occur.
Wernicke’s disease. Initially, Wernicke’s disease produces an intention tremor accompanied by a sluggish pupillary reaction. Later, pupils may become nonreactive. Additional ocular findings include diplopia, gaze paralysis, nystagmus, ptosis, decreased visual acuity, and conjunctival injection. The patient may also exhibit orthostatic hypotension, tachycardia, ataxia, apathy, and confusion.
Special Considerations
A sluggish pupillary reaction isn’t diagnostically significant, although it occurs with various disorders.
Patient Counseling
Stress the importance of regular ophthalmic examinations. Explain ways to reduce photophobia, and teach the patient self-care for diabetes, if needed.
Pediatric Pointers
Children experience sluggish pupillary reactions for the same reasons as adults.
REFERENCES
Biswas, J. , Krishnakumar, S., & Ahuja, S. (2010) . Manual of ocular pathology. New Delhi, India: Jaypee—Highlights Medical Publishers.
Eagle, R. C. Jr. (2011). Eye pathology: An atlas and text. Philadelphia, PA: Lippincott Williams & Wilkins. Gerstenblith, A. T., & Rabinowitz, M. P. (2012). The wills eye manual. Philadelphia, PA: Lippincott Williams & Wilkins. Roy, F. H. (2012). Ocular differential diagnosis. Clayton, Panama: Jaypee—Highlights Medical Publishers, Inc.
Purpura
Purpura is the extravasation of red blood cells from the blood vessels into the skin, subcutaneous tissue, or mucous membranes. It’s characterized by discoloration that’s easily visible through the epidermis, usually purplish or brownish red. Purpuric lesions include petechiae, ecchymoses, and
hematomas. (See Identifying Purpuric Lesions.) Purpura differs from erythema in that it doesn’t blanch with pressure because it involves blood in the tissues, not just dilated vessels.
Purpura results from damage to the endothelium of small blood vessels, a coagulation defect, ineffective perivascular support, capillary fragility and permeability, or a combination of these factors. These faulty hemostatic factors, in turn, can result from thrombocytopenia or another hematologic disorder, an invasive procedure, or, of course, the use of an anticoagulant.
Additional causes are nonpathologic. Purpura can be a consequence of aging, when loss of collagen decreases connective tissue support of upper skin blood vessels. In an elderly or cachectic person, skin atrophy and inelasticity and loss of subcutaneous fat increase susceptibility to minor trauma, causing purpura to appear along the veins of the forearms, hands, legs, and feet. Prolonged coughing or vomiting can produce crops of petechiae in loose face and neck tissue. Violent muscle contraction, as occurs in seizures or weight lifting, sometimes results in localized ecchymoses from increased intraluminal pressure and rupture. A high fever, which increases capillary fragility, can also produce purpura.
History and Physical Examination
Ask the patient when he first noticed the lesion and whether he has noticed other lesions on his body. Does he or his family have a history of bleeding disorders or easy bruising? Find out what medications he’s taking, if any, and ask him to describe his diet. Ask about recent trauma or transfusions and the development of associated signs, such as epistaxis, bleeding gums, hematuria, and hematochezia. Also, ask about systemic complaints that may suggest infection, such as a fever. If the patient is female, ask about heavy menstrual flow.
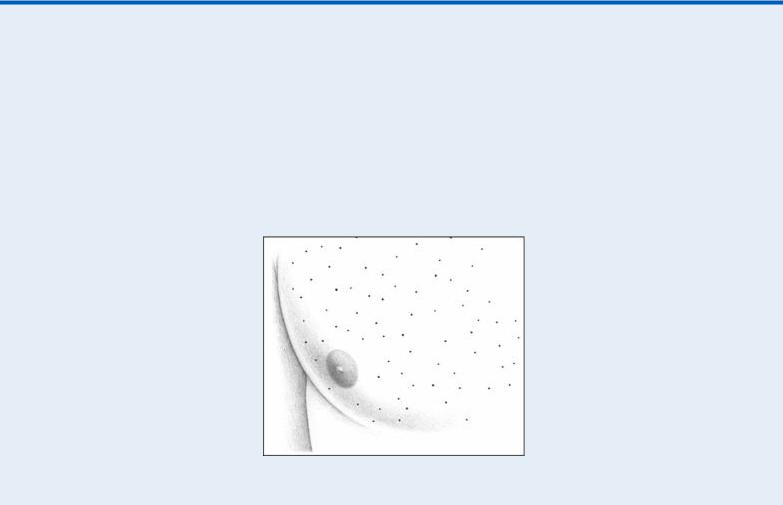
Identifying Purpuric Lesions
Purpuric lesions fall into three categories: petechiae, ecchymoses, and hematomas.
PETECHIAE
Petechiae are painless, round, pinpoint lesions, 1 to 3 mm in diameter. Caused by extravasation of red blood cells into cutaneous tissue, these red or brown lesions usually arise on dependent portions of the body. They appear and fade in crops and can group to form ecchymoses.
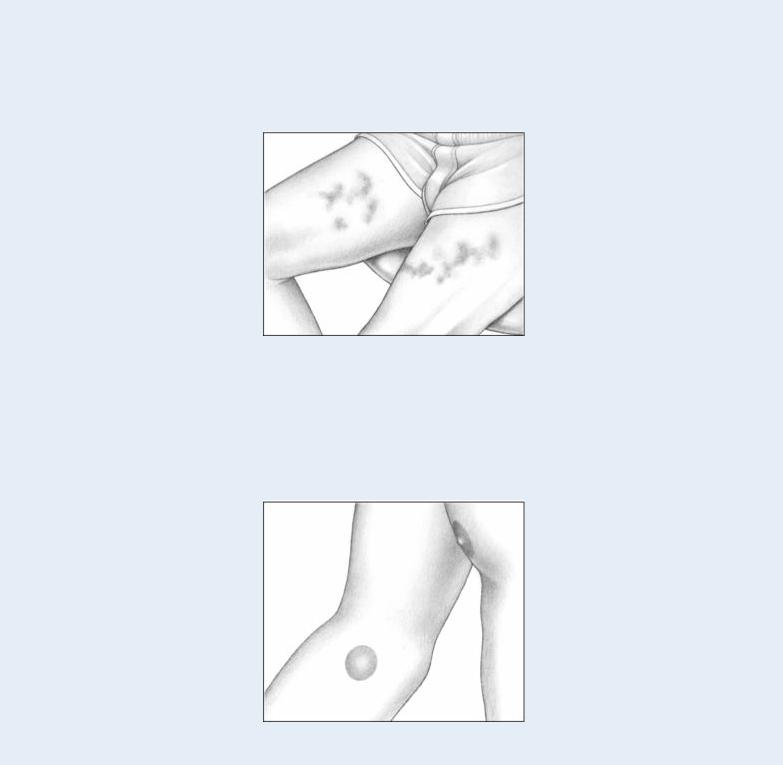
ECCHYMOSES
Ecchymoses, another form of blood extravasation, are larger than petechiae. These purple, blue, or yellow-green bruises vary in size and shape and can arise anywhere on the body as a result of trauma. Ecchymoses usually appear on the arms and legs of patients with bleeding disorders.
HEMATOMAS
Hematomas are palpable ecchymoses that are painful and swollen. Usually the result of trauma, superficial hematomas are red, whereas deep hematomas are blue. Hematomas commonly exceed 1 cm in diameter, but their size varies widely.
 GENDER CUE
GENDER CUE
Purpura is more common in women and particularly in individuals with large areas of subcutaneous fat, such as the breasts, abdomen, buttocks, thighs, and calves.
Inspect the patient’s entire skin surface to determine the type, size, location, distribution, and severity of purpuric lesions. Also, inspect the mucous membranes. Remember that the same mechanisms that cause purpura can also cause internal hemorrhage, although purpura isn’t a cardinal indicator of this condition.
Medical Causes
