
- •1. Heating a house
- •1.1 Transfer of heat
- •1.2 Heat losses
- •1.3 Conduction heat loss
- •1.4 Convection heat loss
- •1.5 Radiation losses
- •1.6 Seasonal heating
- •1.7 Choice of installation
- •2. Investment
- •2.1 The value of money
- •3. Growth of money
- •3.1 Growth of a fixed amount
- •3.2 Growth of regular investment
- •4. Hire purchase
- •5. Buying a house
- •6. Income tax
- •6.1 Allowances
- •6.2 Taxable income
- •6.3 Standard rate and reduced rates of tax
- •6.4 Tax deducted by employer
- •7. Rates
- •7.1 Rateable value
- •7.2 Lp rate
- •7.3 The rates
1.4 Convection heat loss
Do Exercise B before continuing with this sub-section.
Exercise B
-
How much air flows up the chimney per hour from an open fire if the speed of the flow is 1 m/s and the chimney opening is 0-3 m x 0-1 m?
-
How much air flows per hour through a 0-01 m gap under a door 0-8 m wide if it flows at 6 m/s in a high wind? (Think of the feel of the air against you when walking or cycling, to estimate its speed.)
-
How much air flows per hour through an open window 1 m×0-4 m at 1 m/s?
-
If the flows in Questions 1-3 occur in a room 5m×4 m by 2-5 m high, how many times would you estimate the air in the room changes per hour in each case?
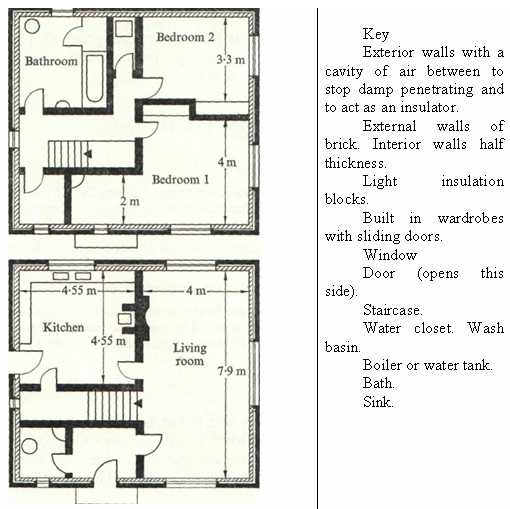
Fig. 1.1 Dwelling house Scale 1cm represents 1-43cm
Air flows into a house through open windows, gaps between floor boards around ill-fitting doors, etc., and out through chimneys, ventilators and windows. Warmed air rises, and the air in a house must be warmed for comfort. The flow of warmed air through a house is known as convection and the warm air that flows out causes convection loss of heat. It is difficult to estimate the total flow of air through a house; in an exposed site there may be (on the average) 3 changes of air per hour throughout the house and in a sheltered site, 2 changes.
Example 3
How much heat per hour will be required to heat the air flowing through a house 9-1m×7-6m ×5-2m in which there are 3 air changes per hour and the average difference of inside and outside temperature is 10 degC?
F = 9-l×7-6×5-15m3,
so L = 1-4× (9-1×7-6×5-2) ×3×10 = 14900 mj/h.
Exercise C
-
Estimate the total heat loss per hour by convection from a building 2mx6mx5m, if there are 2 air changes per hour and the difference between inside and outside temperatures is 10 degC.
-
Estimate: {a) the convection loss; (b) the conduction loss of heat from your classroom per hour.
-
Repeat Question 2 but for your house, under the same temperature conditions.
-
When a heating appliance is bought it should be able to supply sufficient heat to meet the worst reasonable conditions. How many kJ/h would be needed to meet a temperature difference of 20 degC instead of the 10 degC allowed for in Questions 2 and 3? What percentage increase is this?
1.5 Radiation losses
These are usually small and ignored. More heat, in fact, is usually gained than lost by radiation, from solar radiation through south facing windows which during the winter may let in 140 kJ/m2. Cooking also supplies heat and so does each person— about 200 kJ/h. A net gain from all these effects is often assumed for a small house, to be 3000 kJ/h.
1.6 Seasonal heating
To decide on the best way to heat a house, it is necessary to know how much a whole season of heating will cost. There are two new questions here. What is the average temperature difference over the whole season? What is the length of a heating season?
The temperature difference will depend upon the temperature at which you like to keep the house (what is that?) and upon the average outside temperature. In London, for example, the latter may be assumed to be 6 °C during the colder months.
In England, the heating season is taken to be 33 weeks, that is 5544 hours. 5544 is far too precise a figure for such a variable quantity as an English winter. Take 5000 or 6000 according to the district in which you live. Calculations are based upon 6000 for this chapter. A kilojoule is too small a unit for convenient use in a whole season and 1 Megajoule (MJ) is used instead.
Below there are values representing approximate heat supplied by different fuels if fully burnt or used.
Coal or coke 1 tonne produces 28000 MJ
Anthracite 1 tonne produces 34000 MJ
Gas 1 m3 produces 20 MJ
Oil 1l produces 40 MJ
Electricity 1 MJ produces 1 MJ, or
1 kWh produces 3-6 MJ
A unit of electricity is equivalent to the electrical energy used when a 1 kilowatt fire is switched on for an hour; it is sometimes called a kilowatt-hour (kWh).
In the case of an electric fire, all the heat produced goes directly into the house, but, for example, when coal is burnt, part of the heat escapes uselessly up the chimney; the amount which escapes depends upon the efficiency of the appliance. The term house efficiency (h.e.) is used for the percentage of available heat that goes directly into the house.
Figure 1.2 is a nomogram from which the heat loss in MJ on the upright scale can be converted into the heat requirements for differing house efficiencies and then directly below to the actual quantity of fuel required. For example, suppose 90000 MJ is the heat loss; following the dotted line across, for a central heating system of 70 % efficiency, 128000 MJ must be supplied; this would require 3-7 tonnes of anthracite, or 4-6 tonnes of coke, or 3200 1 of oil, or 6400 m3 of gas, or 128000 MJ of electricity.

Fig. 1.2
