

Growth factors: setting the framework
Platelet-derived growth factor (PDGF)
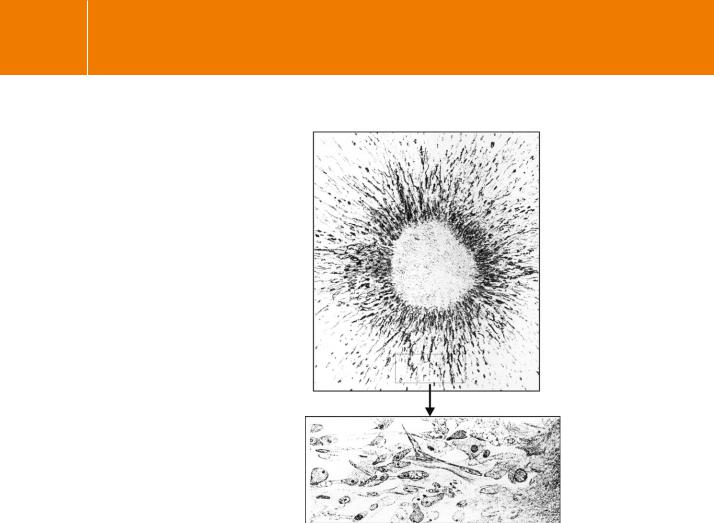
In 1912 Alexis Carrel23 reported a number of experiments having the purpose ‘to determine the conditions under which the active life of a tissue outside that of the organism could be prolonged indefinitely’. When he tried to maintain tissues for a few days in a simple buffered salt solution, the cells lost their growth capacity and then their viability. It was supposed that the senility and death of the cultures was due to the accumulation of catabolic substances and exhaustion of essential nutrients. Continuous and more rapid growth was achieved by supplementing the solution with diluted plasma, and then, from time to time, submerging the tissue in serum for a few hours. Interestingly, the notion that the cells might require specific factors present in the serum never appears to have crossed Carrel’s mind. In conclusion, he wrote that
fragments of connective tissue have been kept in vitro in a condition of active life for more than two months. As a few cultures are now eighty-five days old and are growing very actively, it is probable that, if no accident occurs, the life of these cultures will continue for a long time (Figure 11.3).
About 40 years later, Temin and Dulbecco, working independently, set out to define the precise requirements for cell culture with respect to amino acids, vitamins, salts (together, Carrel’s ‘nutrients’) and importantly, growth factors. Their ambition to culture cells arose from their interest in the role of viruses in cell transformation and tumour formation. Thus an important landmark was the finding that the requirement for serum is drastically reduced in cells infected with tumour viruses. They proposed that transformation might occur as a result of the enhanced capacity of tumour cells to respond to the proliferation signals present in the serum.
An important turning point was the discovery that serum (the soluble component of clotted blood) supports growth and proliferation. Plasma merely allows survival, and over a period of about 2 days cells become quiescent (arrested at the G0 stage of the cell cycle). Serum contains the products of activated platelets, suggesting that they might have a necessary role in the provision of growth factors. In 1974, Russell Ross showed that factors extracted from platelets can induce quiescent smooth muscle cells to synthesize DNA,24,26 and in the same year, Kohler and Lipton obtained
a similar result with mouse 3T3 fibroblasts. The factor derived from blood platelets (platelet-derived growth factor, PDGF) could propel the quiescent cells into the cell cycle S-phase (DNA synthesis).
With the purification of PDGF from human platelets27 the question of how it acts could be faced. PDGF exists as a disulfide-linked dimer, so that binding automatically causes the cross-linking of two receptors. This constitutes the signal for activation.28 Subsequently, it was found that the oncogene of the simian sarcoma virus (v-sis) is homologous to the gene coding for PDGF.29–31
Alexis Carrel was awarded the Nobel Prize (in 1912) in recognition of his work on vascular suture and the transplantation of blood vessels and organs’. He tarnished his reputation by his association
with the eugenics movement, calling for the establishment of institutions equipped with ‘appropriate gases’ in order to eliminate the insane. He gave enthusiastic support to the Vichy government in France during World War II and after the liberation he was charged for collaboration with the Nazi occupiers. He died before his case came to trial. Even his claim to have kept heart cells in culture may have been unwittingly fraudulent. Assistants may have introduced fresh cells
from time to time in order to achieve the expected result . . . and to keep Alexis happy.25
Howard Temin and Renato Dulbecco shared the Nobel prize in 1975 for their discoveries concerning the interaction between tumour viruses and the genetic material of the cell.
303
Signal Transduction
FIG 11.3 ‘A fifty day old culture of connective tissue. Active tissue is encircling a piece of old plasma.’ From Carrel.23
Here was another clear link between a growth factor and a tumour virus. This time, however, the signal to uncontrolled cell proliferation is due to excessive production of growth factor, rather than expression of a constitutively activated receptor. Furthermore, v-sis causes cell transformation in primates.32
The mechanisms by which an oncogene might cause a tumour were becoming clearer. Here is another example of a mammalian gene, surreptitiously borrowed by a virus, then mutated or mutilated. On return to the host by infection, it causes cell transformation and tumour formation.
Transforming growth factors (TGF and TGF )
The transforming growth factors (TGFs) were originally isolated from the conditioned medium of a virally infected mammalian fibroblast cell line, 3T3. These are proteins that can bring about transformation of phenotype.
304

Growth factors: setting the framework
The discovery of the TGFs followed some years after the first reports and descriptions of EGF, and it derives particularly from the work undertaken in the laboratory of George Todaro.33
TGF , although quite distinct from EGF with respect to amino acid sequence, binds to the EGF receptor and signals cells in a similar fashion (see Chapter 12). A related factor isolated from these tumour cells, TGF , which does not compete with the binding of TGF or EGF, can nevertheless induce transformation when provided with either of these two factors. Importantly, TGF is a normal cellular product and the finding of high quantities in blood platelets and its release during blood coagulation established a clear link with PDGF.34
In screening the transforming effect of TGF in numerous tumours, there was an unexpected finding. Depending on the cells and the conditions, TGF can either promote or suppress cell growth and transformation. It cooperates with TGF and EGF to cause cell transformation. On the other hand, it inhibits colony formation in cells derived from human tumours. It appears that its effects
are a function of the total set of growth factors and their receptors that are operational at a given time.35 In addition, TGF plays a number
of key roles in the process of tissue remodelling and wound healing.36 It induces the production of fibronectin and collagen and thus regulates the deposition of the cell matrix, itself a key determinant of cell growth (see page 397).
Problems with nomenclature
As must be evident, nomenclature in this area is arbitrary, to say the least. Some growth factors were named after the cells from which they were first isolated, others from the cells which they stimulated, yet others from the principal action that they appeared to perform.37 In immunology we hear of interleukins and colony stimulating factors. These direct the maturation and proliferation of white blood cells. In virology, we have the interferons that ‘interfere’ with viral infection, and in cancer research we have tumour necrosis factor (and its relatives) that can influence the growth of solid tumours. In each discipline it seemed that these factors functioned mainly in the category in which they first came to light. Of course, we now know that some growth factors have actions that are totally unrelated to growth. For instance, PDGF, released from platelets at sites of tissue damage,38 not only supports the growth of fibroblasts, smooth muscle cells and glial cells, but also acts to regulate the distribution and migration of vascular smooth muscle cells and fibroblasts in wound healing.39
To add further complexity to an already complex situation, the conditions in which the cells are studied can determine the cellular response, for instance the presence of other factors, other cells and attachment to substrates.
A good example of this is TGF . As its name implies, this emerged as a factor enhancing cell transformation, but we now recognize that it can inhibit cell
Cytokines: ‘soluble (glyco)proteins, nonimmunoglobulin in nature, released by living cells of the host, acting non-enzymatically in picomolar to nanomolar concentrations to regulate host cell function.’
305
