
- •Overview
- •Preface
- •Translator’s Note
- •Contents
- •1. Fundamentals
- •Microscopic Anatomy of the Nervous System
- •Elements of Neurophysiology
- •Elements of Neurogenetics
- •General Genetics
- •Neurogenetics
- •Genetic Counseling
- •2. The Clinical Interview in Neurology
- •General Principles of History Taking
- •Special Aspects of History Taking
- •3. The Neurological Examination
- •Basic Principles of the Neurological Examination
- •Stance and Gait
- •Examination of the Head and Cranial Nerves
- •Head and Cervical Spine
- •Cranial Nerves
- •Examination of the Upper Limbs
- •Motor Function and Coordination
- •Muscle Tone and Strength
- •Reflexes
- •Sensation
- •Examination of the Trunk
- •Examination of the Lower Limbs
- •Coordination and Strength
- •Reflexes
- •Sensation
- •Examination of the Autonomic Nervous System
- •Neurologically Relevant Aspects of the General Physical Examination
- •Neuropsychological and Psychiatric Examination
- •Psychopathological Findings
- •Neuropsychological Examination
- •Special Considerations in the Neurological Examination of Infants and Young Children
- •Reflexes
- •4. Ancillary Tests in Neurology
- •Fundamentals
- •Imaging Studies
- •Conventional Skeletal Radiographs
- •Computed Tomography (CT)
- •Magnetic Resonance Imaging (MRI)
- •Angiography with Radiological Contrast Media
- •Myelography and Radiculography
- •Electrophysiological Studies
- •Fundamentals
- •Electroencephalography (EEG)
- •Evoked potentials
- •Electromyography
- •Electroneurography
- •Other Electrophysiological Studies
- •Ultrasonography
- •Other Ancillary Studies
- •Cerebrospinal Fluid Studies
- •Tissue Biopsies
- •Perimetry
- •5. Topical Diagnosis and Differential Diagnosis of Neurological Syndromes
- •Fundamentals
- •Muscle Weakness and Other Motor Disturbances
- •Sensory Disturbances
- •Anatomical Substrate of Sensation
- •Disturbances of Consciousness
- •Dysfunction of Specific Areas of the Brain
- •Thalamic Syndromes
- •Brainstem Syndromes
- •Cerebellar Syndromes
- •6. Diseases of the Brain and Meninges
- •Congenital and Perinatally Acquired Diseases of the Brain
- •Fundamentals
- •Special Clinical Forms
- •Traumatic Brain injury
- •Fundamentals
- •Traumatic Hematomas
- •Complications of Traumatic Brain Injury
- •Intracranial Pressure and Brain Tumors
- •Intracranial Pressure
- •Brain Tumors
- •Cerebral Ischemia
- •Nontraumatic Intracranial Hemorrhage
- •Infectious Diseases of the Brain and Meninges
- •Infections Mainly Involving the Meninges
- •Infections Mainly Involving the Brain
- •Intracranial Abscesses
- •Congenital Metabolic Disorders
- •Acquired Metabolic Disorders
- •Diseases of the Basal Ganglia
- •Fundamentals
- •Diseases Causing Hyperkinesia
- •Other Types of Involuntary Movement
- •Cerebellar Diseases
- •Dementing Diseases
- •The Dementia Syndrome
- •Vascular Dementia
- •7. Diseases of the Spinal Cord
- •Anatomical Fundamentals
- •The Main Spinal Cord Syndromes and Their Anatomical Localization
- •Spinal Cord Trauma
- •Spinal Cord Compression
- •Spinal Cord Tumors
- •Myelopathy Due to Cervical Spondylosis
- •Circulatory Disorders of the Spinal Cord
- •Blood Supply of the Spinal Cord
- •Arterial Hypoperfusion
- •Impaired Venous Drainage
- •Infectious and Inflammatory Diseases of the Spinal Cord
- •Syringomyelia and Syringobulbia
- •Diseases Mainly Affecting the Long Tracts of the Spinal Cord
- •Diseases of the Anterior Horns
- •8. Multiple Sclerosis and Other Myelinopathies
- •Fundamentals
- •Myelin
- •Multiple Sclerosis
- •Other Demyelinating Diseases of Unknown Pathogenesis
- •9. Epilepsy and Its Differential Diagnosis
- •Types of Epilepsy
- •Classification of the Epilepsies
- •Generalized Seizures
- •Partial (Focal) Seizures
- •Status Epilepticus
- •Episodic Neurological Disturbances of Nonepileptic Origin
- •Episodic Disturbances with Transient Loss of Consciousness and Falling
- •Episodic Loss of Consciousness without Falling
- •Episodic Movement Disorders without Loss of Consciousness
- •10. Polyradiculopathy and Polyneuropathy
- •Fundamentals
- •Polyradiculitis
- •Cranial Polyradiculitis
- •Polyradiculitis of the Cauda Equina
- •Polyneuropathy
- •Fundamentals
- •11. Diseases of the Cranial Nerves
- •Fundamentals
- •Disturbances of Smell (Olfactory Nerve)
- •Neurological Disturbances of Vision (Optic Nerve)
- •Visual Field Defects
- •Impairment of Visual Acuity
- •Pathological Findings of the Optic Disc
- •Disturbances of Ocular and Pupillary Motility
- •Fundamentals of Eye Movements
- •Oculomotor Disturbances
- •Supranuclear Oculomotor Disturbances
- •Lesions of the Nerves to the Eye Muscles and Their Brainstem Nuclei
- •Ptosis
- •Pupillary Disturbances
- •Lesions of the Trigeminal Nerve
- •Lesions of the Facial Nerve
- •Disturbances of Hearing and Balance; Vertigo
- •Neurological Disturbances of Hearing
- •Disequilibrium and Vertigo
- •The Lower Cranial Nerves
- •Accessory Nerve Palsy
- •Hypoglossal Nerve Palsy
- •Multiple Cranial Nerve Deficits
- •12. Diseases of the Spinal Nerve Roots and Peripheral Nerves
- •Fundamentals
- •Spinal Radicular Syndromes
- •Peripheral Nerve Lesions
- •Fundamentals
- •Diseases of the Brachial Plexus
- •Diseases of the Nerves of the Trunk
- •13. Painful Syndromes
- •Fundamentals
- •Painful Syndromes of the Head And Neck
- •IHS Classification of Headache
- •Approach to the Patient with Headache
- •Migraine
- •Cluster Headache
- •Tension-type Headache
- •Rare Varieties of Primary headache
- •Symptomatic Headache
- •Painful Syndromes of the Face
- •Dangerous Types of Headache
- •“Genuine” Neuralgias in the Face
- •Painful Shoulder−Arm Syndromes (SAS)
- •Neurogenic Arm Pain
- •Vasogenic Arm Pain
- •“Arm Pain of Overuse”
- •Other Types of Arm Pain
- •Pain in the Trunk and Back
- •Thoracic and Abdominal Wall Pain
- •Back Pain
- •Groin Pain
- •Leg Pain
- •Pseudoradicular Pain
- •14. Diseases of Muscle (Myopathies)
- •Structure and Function of Muscle
- •General Symptomatology, Evaluation, and Classification of Muscle Diseases
- •Muscular Dystrophies
- •Autosomal Muscular Dystrophies
- •Myotonic Syndromes and Periodic Paralysis Syndromes
- •Rarer Types of Muscular Dystrophy
- •Diseases Mainly Causing Myotonia
- •Metabolic Myopathies
- •Acute Rhabdomyolysis
- •Mitochondrial Encephalomyopathies
- •Myositis
- •Other Diseases Affecting Muscle
- •Myopathies Due to Systemic Disease
- •Congenital Myopathies
- •Disturbances of Neuromuscular Transmission−Myasthenic Syndromes
- •15. Diseases of the Autonomic Nervous System
- •Anatomy
- •Normal and Pathological Function of the Autonomic Nervous System
- •Sweating
- •Bladder, Bowel, and Sexual Function
- •Generalized Autonomic Dysfunction
- •Index

282 15 Diseases of the Autonomic Nervous System
cated in the wall of the target organ; inside the parasympathetic ganglia, the preganglionic fibers form synapses onto the postganglionic neurons. The parasympathetic fibers of cranial nerves III, VII, and IX innervate the smooth musculature and glands of the head, while those of the vagus n. descend in an extensively branched fiber system to innervate the viscera of the throat, thorax, and abdomen, all the way down to the level of the left colic flexure. Beyond this point (the so-called point of Cannon and Böhm), the abdominal and pelvic
viscera are innervated by the sacral portion of the parasympathetic nervous system. The axons of the preganglionic neurons whose cell bodies lie in the lateral horns of the sacral spinal cord reach the periphery by way of the anterior roots or the pelvic nerves. They form synapses onto the postganglionic neurons in the pelvic plexus (= inferior hypogastric plexus) and in the intramural ganglia of the abdominal and pelvic viscera. The anatomy of the parasympathetic nervous system is shown in Fig. 15.3.
Normal and Pathological Function of the Autonomic Nervous System
The sympathetic and parasympathetic nervous systems regulate the functions of the internal organs and are the substrate for all of the autonomic reflexes of the body. The neurotransmitter used at the synapses of the parasympathetic nervous system is acetylcholine, while the sympathetic nervous system uses acetylcholine at the synapse onto the preganglionic neuron, norepinephrine at the synapse onto the postganglionic neuron, and epinephrine in the adrenal cortex (whence the name, epinephrine). An overview of the major functions of the two halves of the autonomic nervous system is provided in Table 15.1.
In the following paragraphs, we will describe just a few, clinically relevant functional disturbances and diseases of the autonomic nervous system.
Sweating
The autonomic fibers innervating the sweat glands are exclusively sympathetic. They run in the peripheral nerves in close association with the somatosensory fibers innervating the same area of skin. A lesion of a peripheral nerve, therefore, always impairs sweating in the sensory distribution of the nerve. The impairment of sweating can be demonstrated with various tests, such as the ninhydrin test (Fig. 15.4).
Fig. 15.4 Ninhydrin test in a right median nerve lesion. The patient lays his hand on a piece of paper that is subsequently “developed” with several applications of a 1 % ninhydrin solution, followed by drying in a hot air chamber. Sweating is diminished or absent in the cutaneous sensory distribution of the median n.
Table 15.1 Functions of the sympathetic and parasympathetic nervous systems
Effect of the sympathetic nervous system |
Organ |
Effect of the parasympathetic nervous |
|||
|
|
|
|
system |
|
|
|
|
|
|
|
|
|
eye: |
|
|
|
+ |
pupillary dilation |
|
dilator pupillae m. |
|
|
|
|
|
sphincter pupillae m. |
+ |
pupillary constriction |
+ vasoconstriction in certain areas of the |
vascular smooth muscle |
− vasodilatation in certain areas of the |
|||
|
body, e. g., the skin |
|
|
|
body, e. g., gastrointestinal tract |
− |
diminished secretion |
salivary glands |
+ |
increased secretion |
|
+ |
increased secretion |
sweat glands |
|
|
|
|
|
lacrimal glands |
+ |
increased secretion |
|
|
|
glands of the GI tract |
+ |
increased secretion |
|
− diminished motility and peristalsis |
smooth muscle of the GI tract |
+ increased motility and peristalsis |
|||
− |
bronchodilation |
bronchial smooth muscle |
+ |
bronchoconstriction |
|
+ |
increased heart rate |
heart |
− |
decreased heart rate |
|
− |
urinary retention |
smooth muscle of the vesical wall |
+ micturition |
||
+ |
urinary retention |
sphincters |
− micturition |
||
|
|
|
|
|
|
Mumenthaler / Mattle, Fundamentals of Neurology © 2006 Thieme All rights reserved. Usage subject to terms and conditions of license.

Normal and Pathological Function of the Autonomic Nervous System 283
Fig. 15.5 Spastic neurogenic bladder. The bladder is cut off from the influence of the CNS above the level of the lesion, but the spinal reflex arc controlling micturition is intact. It is automatically activated whenever the bladder is filled to a certain volume.
 voluntary bladder control
voluntary bladder control
 bladder filling and mural tension
bladder filling and mural tension
 pain and temperature sensation
pain and temperature sensation
site of lesion
S2–4
Bladder, Bowel, and Sexual Function |
which are under voluntary control, is innervated by |
Anatomy. The neural elements controlling bladder, |
the pudendal n., whose fibers are derived from spinal |
cord segments S2−S4. This nerve also conveys affer- |
|
bowel, and sexual function are the following: |
ent impulses arising in the urethra, prostate gland, |
Sympathetic fibers from spinal cord segments T12− |
anal canal, and external genitalia. |
L2 and parasympathetic fibers from spinal cord seg- |
|
ments S2−S4 innervate the smooth muscle of the uri- |
Disturbances of bladder, bowel, and sexual function. |
nary bladder, rectum, and internal genitalia, includ- |
The clinical manifestations depend on the site of the le- |
ing the corpora cavernosa. The sympathetic fibers |
sion (peripheral/central, unilateral/bilateral): |
travel to their target organs after a synaptic relay in |
Spinal cord transection above the sacral level cuts off |
the superior hypogastric plexus, while the parasym- |
the bladder and bowel from the supraspinally |
pathetic fibers do so after a synaptic relay in the infe- |
derived (cortical) impulses subserving the voluntary |
rior hypogastric plexus. There are ganglion cells and |
control of micturition and defecation, but all of the |
synapses not just in the plexuses, but also within the |
afferent and efferent nerve pathways of the bladder |
walls of the target organs. Visceral sensory (afferent) |
remain intact, including the spinal reflex arc for blad- |
fibers return to the spinal cord from the urinary blad- |
der emptying. The result is a spastic (automatic) neu- |
der, genitalia, and rectum. |
rogenic bladder, which empties itself reflexively |
The spinal center for micturition and defecation re- |
whenever it is filled to a certain volume (Fig. 15.5). |
ceives supranuclear input from multiple higher corti- |
Penile erection remains possible, though there may |
cal areas (paracentral lobule voluntary initiation |
be retrograde ejaculation into the bladder. |
of micturition and defecation) through a number of |
|
different pathways in the spinal cord, and it also con- |
Lesions of the conus medullaris, cauda equina, sacral |
veys afferent information back upward to the brain |
plexus, and pelvic plexus. Lesions of these structures |
( conscious perception of bladder filling and of |
inactivate the sacral centers for micturition and defeca- |
noxious and thermal stimuli). These mechanisms are |
tion. The result is atony of the bladder and bowel |
the basis of the voluntary control of micturition and |
musculature, leading to severe impairment of emptying. |
defecation. |
Bladder filling can no longer be perceived, either con- |
The striated skeletal muscle of the pelvic floor and of |
sciously or unconsciously. Tone is preserved in the sym- |
the external sphincters of the bladder and rectum, |
pathetically elevated vesical sphincter; the bladder, |
ARgoBold
ThiemeArgoOne
Mumenthaler / Mattle, Fundamentals of Neurology © 2006 Thieme
All rights reserved. Usage subject to terms and conditions of license.
Diseases of the Autonomic Nervous System
15
284 15 Diseases of the Autonomic Nervous System
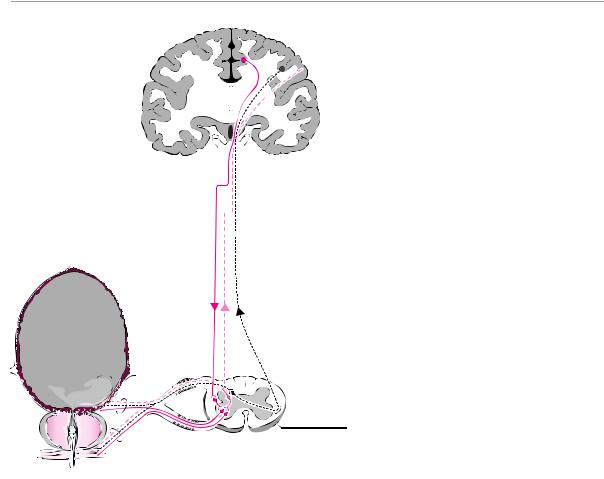
Fig. 15.6 Flaccid neurogenic bladder. The sacral micturition center is destroyed, and the vesical musculature can no longer be induced to contract. The bladder fills until the intravesical pressure exceeds that of the external vesical sphincter; thereafter, urine is released in small quantities at shorter than normal intervals. Complete bladder emptying is no longer possible.
 voluntary bladder control
voluntary bladder control
bladder filling and mural tension
 pain and temperature sensation
pain and temperature sensation
S2–4
site of lesion
therefore, continues to fill until the passive intravesical pressure overcomes the closing force of the sphincter. The continually overfilled bladder lets out small amounts of urine at short intervals (overflow incontinence, Fig. 15.6). Defecation, meanwhile, occurs passively and in uncontrolled fashion through a patulous anal sphincter. In the male, lesions of these structures cause erectile impotence. Psychosexually mediated arousal remains possible in rare cases because of the preserved sympathetic efferent innervation through the hypogastric plexus. Thus, a small number of affected men are still able to have an emission of semen, but without ejaculation, and without rhythmic contraction of the pelvic floor muscles.
Lesions of the pudendal n. An isolated lesion of the pudendal n., which contains parasympathetic fibers from segments S2−S4, causes erectile dysfunction: the sacral erection center can no longer be activated because its somatosensory afferent input has been interrupted. Moreover, because the somatic efferent impulses to the bulbocavernosus and ischiocavernosus mm. no longer reach their targets, the maximal tumescence of the corpora cavernosa mediated by these muscles also fails to occur.
Impairment of the sympathetic innervation of the pelvic organs can be caused, for example, by tumor infiltration or by surgical procedures. Bilateral lesions of the
sympathetic chain and lesions of the superior hypogastric plexus abolish seminal emission into the proximal urethra; if ejaculation does occur, then the semen goes into the bladder, in retrograde fashion. As long as the parasympathetic innervation of the genital organs by the pelvic plexus and their somatic sensory and motor innervation by the pudendal n. remain intact, the affected men are still able to have erections, and affected persons of both sexes can still experience pelvic floor contractions and orgasm. This constellation of symptoms (preserved ability to experience orgasm, in the absence of seminal emission) is seen in about half of all men who have undergone bilateral sympathectomy. It does not occur after unilateral lumbar sympathectomy.
The Cervical Sympathetic Pathway and
Horner Syndrome
Anatomy. As already discussed at the beginning of this chapter, the spinal cord nuclei in which sympathetic impulses originate are present only from the T2 level downward. Thus, the sympathetic fibers innervating the head must ascend from the thoracic spinal cord and the thoracic segments of the sympathetic chain, by way of the interganglionic branches, to the cervical sympathetic chain, where they make a synaptic relay onto the second neuron in one of the three cervical ganglia (in-
Mumenthaler / Mattle, Fundamentals of Neurology © 2006 Thieme All rights reserved. Usage subject to terms and conditions of license.

Normal and Pathological Function of the Autonomic Nervous System 285
cluding the stellate ganglion). From these ganglia, the sympathetic fibers continue upward in periarterial nerve plexuses until they reach their destinations. Sympathetic fibers in the head innervate the walls of the blood vessels, the sweat glands, and the salivary, lacrimal, nasal, and palatal glands, as well as the smooth muscle of the dilator pupillae m. See also Fig. 15.2, p. 280.
Lesions of the cervical sympathetic pathway. Destruction of the stellate ganglion or of the cervical sympathetic chain causes Horner syndrome: the pupil is (unilaterally) narrow and, when the patient looks slightly downward, ptosis is evident (p. 192). Horner syndrome is usually seen in conjunction with loss of sweating on the ipsilateral upper quadrant of the body, particularly on the neck and face. Depending on the level of the lesion, the arm, hand, and axilla may be affected as well. If the sympathetic chain is interrupted immediately below the stellate ganglion, anhidrosis of the upper quadrant of the body results, but without Horner syndrome. On the other hand, isolated Horner syndrome without anhidrosis can occur as the result of a lesion of the C8−T2 nerve roots between the spinal cord and the sympathetic chain.
Generalized Autonomic Dysfunction
Polyneuropathy. Damage to autonomic fibers is often a component of polyneuropathy. Affected persons suffer from impaired regulation of blood pressure and sweat-
ing, as well as from diarrhea, urinary disturbances, and erectile dysfunction. Autonomic manifestations of these kinds are particularly common in diabetic polyneuropathy.
Acute pandysautonomia. This condition is due to a neuropathy affecting either preganglionic or postganglionic autonomic nerve fibers. Patients suffer from orthostatic hypotension, an invariant heart rate, a lack of sweating and lacrimation, nonreactive midsized pupils, impotence, and an atonic bladder. The etiology of this condition is not known; it gradually resolves spontaneously over the course of a few months.
Familial dysautonomia (Riley). This autosomal recessive disease is probably due to a disturbance of norepinephrine synthesis. Its manifestations, which are already evident in infancy, include dysphagia, lack of tears when the infant cries, abnormally intense sweating, diminished sensitivity to pain, and impaired temperature regulation. The prognosis is poor.
Other generalized autonomic disturbances. A number of degenerative conditions of the basal ganglia can impair autonomic function; further diseases that can affect the autonomic nervous system include orthostatic hypotension of Shy−Drager type, botulinus intoxication, and congenital sensory neuropathy with anhidrosis.
Diseases of the Autonomic Nervous System
15
ARgoBold
ThiemeArgoOne
Mumenthaler / Mattle, Fundamentals of Neurology © 2006 Thieme
All rights reserved. Usage subject to terms and conditions of license.
