
Color Atlas of Physiology 2003 thieme
.pdf



|
Carbohydrate Metabolism and |
glucose concentration as constant as possible |
||||||||||
|
(!A); and (4) promote growth. |
|
|
|||||||||
|
Pancreatic Hormones |
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|||
|
Glucose is the central energy carrier of the |
Insulin |
|
|
|
|
|
|
||||
|
human metabolism. The brain and red blood |
Synthesis. Insulin is a 6 kDa peptide (51 amino acids, |
||||||||||
|
cells are fully glucose-dependent. The plasma |
AA) formed by the C chain cleaved from proinsulin |
||||||||||
|
glucose concentration (blood sugar level) is |
(84 AA), the precursor of which is preproinsulin, a pre- |
||||||||||
|
determined by the level of glucose production |
prohormone. Insulin contains two peptide chains (A |
||||||||||
|
and B) held together by disulfide bridges Degrada- |
|||||||||||
|
and consumption. |
|
||||||||||
|
|
tion: Insulin has a half-life of about 5–8 min and is de- |
||||||||||
|
|
|
|
|||||||||
Reproduction |
The following terms are important for proper under- |
graded mainly in liver and kidneys. |
|
|
||||||||
standing of carbohydrate metabolism (!A, C): |
Secretion. |
Insulin |
is |
secreted in pulsatile |
||||||||
|
||||||||||||
|
1. Glycolysis generally refers to |
the anaerobic |
||||||||||
|
bursts, mainly in response to increases in the |
|||||||||||
|
conversion of glucose to lactate (!p. 72). This oc- |
|||||||||||
|
blood levels of glucose (!B right), as fol- |
|||||||||||
|
curs in the red blood cells, renal medulla, and skeletal |
|||||||||||
|
muscles (!p. 72). Aerobic oxidation of glucose oc- |
lows: plasma glucose "!glucose in B cells |
||||||||||
and |
curs in the CNS, heart, skeletal muscle and in most |
"!glucose oxidation "!cytosolic ATP |
||||||||||
other organs. |
|
|
"!closure |
of |
ATP-gated K+ |
channels |
||||||
Hormones |
2. Glycogenesis, i.e., the synthesis of glycogen |
!depolarization !opening of voltage-gated |
||||||||||
muscle can only be used by that muscle. |
and (b) re-opening of K+ channels (deactivated |
|||||||||||
|
from glucose (in liver and muscle), facilitates the |
Ca2+ |
channels |
!cytosolic Ca2+". The rising |
||||||||
|
storage of glucose and helps to maintain a constant |
Ca2+ in B cells leads to (a) exocytosis of insulin |
||||||||||
|
plasma glucose concentration. Glycogen stored in a |
|||||||||||
|
|
|
|
|
|
|
|
|
||||
11 |
3. Glycogenolysis is the breakdown of glycogen |
by |
feedback |
control). |
Stimulation. |
Insulin |
||||||
to glucose, i.e., the opposite of glycogenesis. |
secretion is stimulated mainly during food |
|||||||||||
|
||||||||||||
|
4. Gluconeogenesis is the production of glucose |
digestion via acetylcholine (vagus nerve), |
||||||||||
|
(in liver and renal cortex) from non-sugar molecules |
gastrin, secretin, |
GIP |
(!p. 234) |
and |
GLP-1 |
||||||
|
such as amino acids (e.g., glutamine), lactate (pro- |
(glucagon-like |
peptide |
= enteroglucagon), a |
||||||||
|
duced by anaerobic glycolysis in muscles and red |
|||||||||||
|
peptide that dissociates from intestinal pro- |
|||||||||||
|
cells), and glycerol (from lipolysis). |
|
||||||||||
|
|
glucagon. Certain amino acids (especially ar- |
||||||||||
|
5. Lipolysis is the breakdown of triacylglycerols |
|||||||||||
|
ginine and leucine), free fatty acids, many |
|||||||||||
|
into glycerol and free fatty acids. |
|
||||||||||
|
6. Lipogenesis is the synthesis of triacylglycerols |
pituitary hormones and some steroid hor- |
||||||||||
|
(for storage in fat depots). |
|
mones also increase insulin secretion. Inhibi- |
|||||||||
|
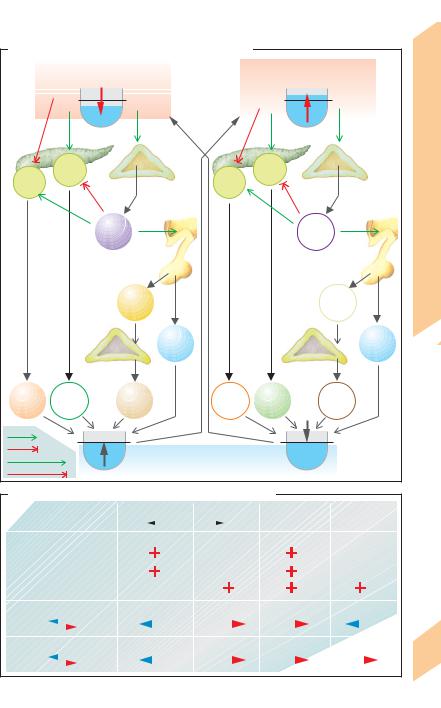
Islets of Langerhans in the pancreas play a pri- |
tion. Epinephrine |
and |
norepinephrine (α2- |
||||||||
|
adrenoceptors; !A, B), SIH (!p. 273 B) and |
|||||||||||
|
mary role in carbohydrate metabolism. Three |
|||||||||||
|
the neuropeptide galanin inhibit insulin secre- |
|||||||||||
|
cell types (A, B, D) have been identified so far |
|||||||||||
|
tion. When hypoglycemia occurs due, e.g., to |
|||||||||||
|
(!p. 273 B). 25% of all islet cells are type A (α) |
|||||||||||
|
fasting or prolonged physical exercise, the low |
|||||||||||
|
cells that produce glucagon, 60% are B (") cells |
|||||||||||
|
blood glucose concentration is sensed by cen- |
|||||||||||
|
that synthesize insulin, and 10% are D (δ) cells |
|||||||||||
|
tral chemosensors for glucose, leading to reflex |
|||||||||||
|
that secrete |
somatostatin (SIH). These hor- |
||||||||||
|
activation of the sympathetic nervous system. |
|||||||||||
|
mones mutually influence the synthesis and |
|||||||||||
|
|
|
|
|
|
|
|
|
||||
|
secretion of each other (!p. 273 B). Islet cells |
The insulin receptor is a heterotetramer (α2"2) con- |
||||||||||
|
in the pancreas head synthesize pancreatic |
sisting of two extracellular α subunits and two trans- |
||||||||||
|
polypeptide, |
the physiological |
function of |
membranous " subunits. The α subunits bind the |
||||||||
|
which is not yet clear. High concentrations of |
hormone. Once the " subunits are autophosphory- |
||||||||||
|
lated, they |
act |
as |
receptor tyrosine |
kinases that |
|||||||
|
these hormones reach the liver by way of the |
|||||||||||
|
phosphorylate insulin receptor substrate-1 (IRS-1). In- |
|||||||||||
|
portal venous circulation. |
|
||||||||||
|
|
tracellular proteins with SH2 domains are phospho- |
||||||||||
|
Function. Pancreatic hormones (1) ensure |
|||||||||||
|
rylated by IRS-1 and pass on the signal (!p. 277 C3). |
|||||||||||
|
that ingested food is stored as glycogen and fat |
Action of insulin (!A, B, C). Insulin has ana- |
|
|
(insulin); (2) mobilize energy reserves in re- |
||
|
bolic and lipogenic effects, and promotes the |
||
|
sponse to food deprivation, physical activity or |
||
|
storage of glucose, especially in the liver, where |
||
282 |
stress (glucagon and the non-pancreatic hor- |
||
it activates enzymes that promote glycolysis |
|||
mone epinephrine); (3) maintain the plasma |
|||
|
|||
|
|
!
Despopoulos, Color Atlas of Physiology © 2003 Thieme
All rights reserved. Usage subject to terms and conditions of license.



|
Thyroid Hormones |
thyrocolloid. The phenol ring of one DIT (or |
|
|
MIT) molecule links with another DIT |
||
|
|
||
|
The thyroid gland contains spherical follicles |
molecule (ether bridges). The resulting thyro- |
|
|
(50–500 µm in diameter). Follicle cells synthe- |
globulin chain now contains tetraiodothy- |
|
|
size the two iodine-containing thyroid hor- |
ronine residues and (less) triiodothyronine resi- |
|
|
mones thyroxine (T4, tetraiodothyronine) and |
dues (!C). These are the storage form of T4 and |
|
|
triiodothyronine (T3). T3 and T4 are bound to |
T3. |
|
|
the glycoprotein thyroglobulin (!B2) and |
TSH also stimulates T3 and T4 secretion. The |
|
|
stored in the colloid of the follicles (!A1, B1). |
iodinated thyroglobulin in thyrocolloid are re- |
|
Reproduction |
The synthesis and release of T3/T4 is controlled |
absorbed by the cells via endocytosis (!B3, |
|
by the thyroliberin (= thyrotropin-releasing |
C). The endosomes fuse with primary lyso- |
||
|
|||
|
hormone, TRH)—thyrotropin (TSH) axis (!A, |
somes to form phagolysosomes in which thy- |
|
|
and p. 270ff.). T3 and T4 influence physical |
roglobulin is hydrolyzed by proteases. This |
|
|
growth, maturation and metabolism. The par- |
leads to the release of T3 and T4 (ca. 0.2 and |
|
|
afollicular cells (C cells) of the thyroid gland |
1–3 mol per mol of thyroglobulin, respec- |
|
and |
synthesize calcitonin (!p. 292). |
tively). T3 and T4 are then secreted into the |
|
Thyroglobulin, a dimeric glycoprotein (660 kDa) is |
bloodstream (!B3). With the aid of deiodase, |
||
Hormones |
I– meanwhile is split from concomitantly re- |
||
synthesized in the thyroid cells. TSH stimulates the |
|||
|
|||
|
leased MIT and DIT and becomes reavailable |
||
|
transcription of the thyroglobulin gene. Thyro- |
||
|
for synthesis. |
||
|
globulin is stored in vesicles and released into the col- |
||
|
loid by exocytosis (!B1 and p. 30). |
Control of T3/T4 secretion. TSH secretion by |
|
11 |
Iodine uptake. The iodine needed for hormone |
the anterior pituitary is stimulated by TRH, a |
|
hypothalamic tripeptide (!p. 280) and in- |
|||
|
synthesis is taken up from the bloodstream as |
||
|
hibited by somatostatin (SIH) (!A and p. 270). |
||
|
iodide (I–). It enters thyroid cells through sec- |
||
|
The effect of TRH is modified by T4 in the |
||
|
ondary active transport by the Na+-I– symport |
||
|
plasma. As observed with other target cells, |
||
|
carrier (NIS) and is concentrated in the cell ca. |
||
|
the T4 taken up by the thyrotropic cells of the |
||
|
25 times as compared to the plasma (!B2). |
||
|
anterior pituitary is converted to T3 by 5!-deio- |
||
|
Via cAMP, TSH increases the transport capacity |
||
|
dase. T3 reduces the density of TRH receptors in |
||
|
of basolateral I– uptake up to 250 times. Other |
||
|
the pituitary gland and inhibits TRH secretion |
||
|
anions competitively inhibit I– uptake; e.g., |
||
|
by the hypothalamus. The secretion of TSH and |
||
|
ClO4–, SCN– and NO2–. |
||
|
consequently of T3 and T4 therefore decreases |
||
|
Hormone synthesis. I– ions are continuously |
||
|
(negative feedback circuit). In neonates, cold |
||
|
transported from the intracellular I– pool to the |
||
|
seems to stimulate the release of TRH via neu- |
||
|
apical (colloidal) side of the cell by a I–/Cl– anti- |
||
|
ronal pathways (thermoregulation, !p. 224). |
||
|
porter, called pendrin, which is stimulated by |
||
|
TSH is a heterodimer (26 kDa) consisting of an |
||
|
TSH. With the aid of thyroid peroxidase (TPO) |
||
|
α subunit (identical to that of LH and FSH) and |
||
|
and an H2O2 generator, they are oxidized to el- |
||
|
a " subunit. TSH controls all thyroid gland func- |
||
|
ementary I20 along the microvilli on the colloid |
||
|
tions, including the uptake of I–, the synthesis |
||
|
side of the cell membrane. With the help of |
||
|
and secretion of T3 and T4 (!A-C), the blood |
||
|
TPO, the I0 reacts with about 20 of the 144 ty- |
||
|
flow and growth of the thyroid gland. |
||
|
rosyl residues of thyroglobulin (!C). The |
||
|
|
||
|
phenol ring of the tyrosyl residue is thereby |
Goiter (struma) is characterized by diffuse or nodu- |
|
|
iodinated at the 3 and/or 5 position, yielding a |
lar enlargement of the thyroid gland. Diffuse goiter |
|
|
protein chain containing either diiodotyrosine |
can occur due to an iodine deficiency, resulting in |
|
|
(DIT) residues and/or monoiodotyrosine (MIT) |
T3/T4 deficits that ultimately lead to increased secre- |
|
|
tion of TSH. Chronic elevation of TSH leads to the |
||
|
residues. These steps of synthesis are stimu- |
||
|
proliferation of follicle cells, resulting in goiter |
||
|
lated by TSH (via IP3) and inhibited by |
||
|
development (hyperplastic goiter). This prompts an |
||
|
thiouracil, thiocyanate, glutathione, and other |
||
|
increase in T3/T4 synthesis, which sometimes nor- |
||
|
reducing substances. The structure of the thy- |
malizes the plasma concentrations of T3/T4 (euthyroid |
|
286 |
roglobulin molecule allows the iodinated ty- |
goiter). This type of goiter often persists even after |
|
|
rosyl residues to react with each other in the |
the iodide deficiency is rectified. |
|
|
|
! |
Despopoulos, Color Atlas of Physiology © 2003 Thieme
All rights reserved. Usage subject to terms and conditions of license.



