

Supplement A3: The Chemistry of Double-Bonded Functional Groups. Edited by Saul Patai Copyright 1997 John Wiley & Sons, Ltd.
ISBN: 0-471-95956-1
CHAPTER 10
Pyrolysis involving compounds with C=C, C=N and C=O double bonds
R. ALAN AITKEN and ANDREW W. THOMAS
School of Chemistry, University of St. Andrews, St. Andrews, Fife, KY16 9ST, UK Fax: 44-1334-463-808; e-mail: RAA@ST-AND.AC.UK
I. INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
474 |
|
II. COMPOUNDS CONTAINING CDC DOUBLE BONDS . . . . . . . . . . . . |
475 |
|
A. Retro-Diels Alder Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
475 |
|
B. Retro-ene Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
479 |
|
C. Miscellaneous Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
480 |
|
III. COMPOUNDS CONTAINING CDN DOUBLE BONDS . . . . . . . . . . . . |
484 |
|
A. Imines, Oximes and Hydrazones . . . . . . . . . . . . . . . . . . . . . . . . . . |
484 |
|
B. Pyrazoles and Indazoles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
485 |
|
C. Isoxazoles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
489 |
|
D. Triazoles and Tetrazoles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
490 |
|
E. Rings Containing Sulphur or Phosphorus . . . . . . . . . . . . . . . . . . . . |
491 |
|
F. Pyrimidines and Diazepines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
491 |
|
IV. COMPOUNDS CONTAINING CDO DOUBLE BONDS . . . . . . . . . . . . |
493 |
|
A. Aldehydes and Ketones . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
493 |
|
B. Esters and Thioesters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
494 |
|
C. Acid Chlorides and Amides . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
501 |
|
D. Acyl Phosphorus Ylides . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
503 |
|
E. Acid Anhydrides . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
507 |
|
F. Isoxazolones and Oxazolones . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
512 |
|
G. Meldrum’s Acid Derivatives . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
516 |
|
H. Cyclic 1,2-Diketones . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
524 |
|
1. |
Cyclobutenediones . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
524 |
2. |
Furan-2,3-diones and thiophene-2,3-diones . . . . . . . . . . . . . . . . . |
525 |
3. |
Pyrrole-2,3-diones and pyrazole-3,4-diones . . . . . . . . . . . . . . . . . |
526 |
473

474 |
R. Alan Aitken and Andrew W. Thomas |
|
I. |
1,3-Dioxinones and 1,3-Dioxinthiones . . . . . . . . . . . . . . . . . . . . . . |
527 |
J.Rings Containing Sulphur . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 528
K.Miscellaneous Heterocyclic Compounds . . . . . . . . . . . . . . . . . . . . . 530
V. REFERENCES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
530 |
I. INTRODUCTION
In the early days of organic chemistry the action of heat alone on a newly isolated compound was often investigated as a means of obtaining information on structure and reactivity, and many important transformations were discovered in this way. In his monumental work of 1929, Hurd1 made an attempt to collate all pyrolytic reactions reported up to that time according to the functional groups involved. Over the next 50 years only a few research groups were active in developing preparative pyrolytic methods and most of the work was concerned with gas-phase kinetic measurements. In the last 25 years there has been a great resurgence of interest in ‘pyrolytic methods in organic chemistry’ and this owes a lot to the monograph with this title published by Brown2 in 1980. In this, the various experimental setups available for performing pyrolysis reactions were described, together with a detailed survey of the most important reaction types.
The technique of flash vacuum pyrolysis (FVP) has now become widely accepted by specialists as being the method of choice for carrying out many types of unimolecular thermal reaction. In more general circles, however, there are still some misapprehensions which limit its more widespread adoption. The first is that it involves extremely severe conditions and is likely to cause fragmentation, racemization or complete destruction of any reasonably complex synthetic intermediate. This arises in part from the apparently high temperatures involved (350 >1000 °C), but it must be remembered that, at the low pressures commonly used (10 1 10 6 torr), each molecule only spends a short time in contact with the hot zone. In reality the whole range of activation energies for reaction is accessible including the mildest of conditions (250 400 °C, 10 3 torr) corresponding to heating in solution at little above room temperature. A second common perception is that the method is only useful for generating highly reactive products in minute quantities for spectroscopic detection. While studies of this type have been made, FVP is also useful for preparing multi-gram quantities of products for further use. So long as the success of a reaction does not depend critically on having a very low pressure, there is no reason why it cannot be scaled up to multi-kilogram scale and beyond. As is shown by many of the examples later in this chapter, pyrolytic methods in general and FVP in particular are now finding increasing use, both in the routine preparation of often sensitive compounds not readily accessible by other methods and to carry out selected steps in the course of target-directed synthesis.
In this chapter we have attempted to present a representative selection of studies involving the pyrolysis of compounds containing CDC, CDN and CDO groups in the period since 1980. Most of the examples involve reaction in the gas phase in a flow system, but more conventional solution thermolysis is also included. In addition to Brown’s monograph2, a number of previous general reviews of pyrolytic methods contain examples from these compound classes3 5. There have also been more specific reviews on ketenes6,7, ˛-oxoketenes8 and carbenes and alkynes7,9, which contain much valuable information. Although diazo compounds might be considered as containing a CDN group, they have been excluded from this chapter since thermal loss of N2 is such a prominant feature of their chemistry that it is adequately treated in the previous volume of this series devoted to them10.
10.Pyrolysis involving compounds with CDC, CDN and CDO double bonds 475
II. COMPOUNDS CONTAINING C=C DOUBLE BONDS
A. Retro-Diels Alder Reactions
The reverse of the Diels Alder [4 C 2] cycloaddition reaction can be readily achieved by pyrolysis, and since one molecule of starting material generally gives two product molecules it is highly favoured by the low-pressure conditions of FVP. Care must be taken, however, that the two products do not simply undergo cycloaddition again when they are collected. This problem, which is important for reactive 1,3-dienes such as cyclopentadiene, can generally be overcome by collecting the products in separate regions of the cold trap according to their volatility. The retro-Diels Alder reaction was comprehensively reviewed in 197811 and again in 198512 and this section only covers more recent examples.
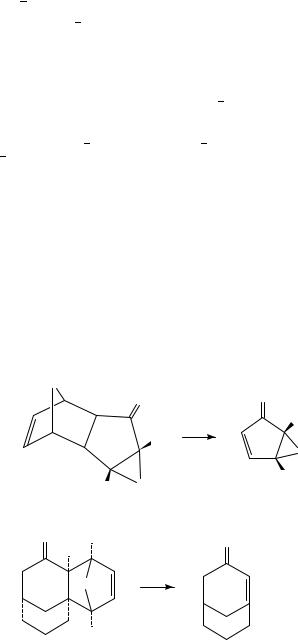
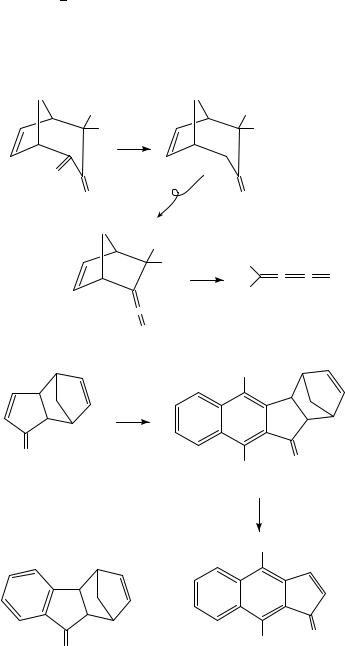
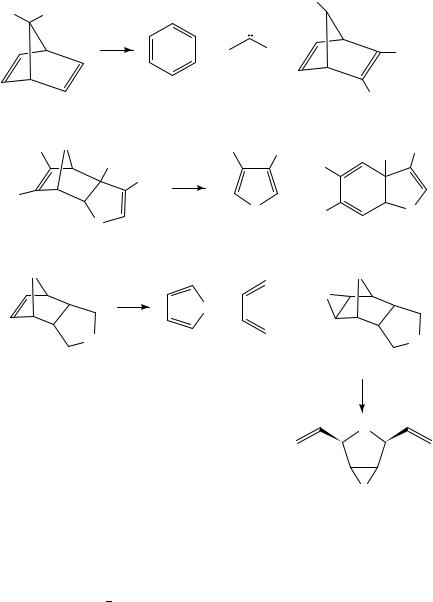
The combination of Diels Alder and retro-Diels Alder reactions can be regarded as a protection deprotection strategy for double bonds and pyrolytic extrusion of a volatile diene such as furan or cyclopentadiene is often the final step in the preparation of reactive alkenes. This has allowed the preparation of the cyclopentadienone monoepoxides 2 by pyrolytic loss of furan from 113, and pyrolysis of 3 gives the strained bicyclic enone 4 which forms a mixture of stereoisomeric [2C2] dimers14. Pyrolysis of 5 at 430 °C and 10 4 torr results in loss of N2 to give the carbene which undergoes a Wolff rearrangement to 6 and finally loses furan to afford the alkylideneketenes 715. Cyclopentadiene has been used to protect one double bond of cyclopentadienone in the adduct 8 which can then undergo benzannulation to give 9, which is converted by FVP to the little known benz[f]indenones 10 by pyrolytic loss of cyclopentadiene16. The benzo analogue 11 has similarly been used as a source of indenone in work directed towards synthesis of kinamycin antibiotics17. FVP of 12 provides access to the cyclopentazulene 1318, and the corresponding enone19 and its hydroxy derivative20 have likewise been generated by FVP at 550 °C of 14 and 15, respectively. Pyrolysis of the chiral adduct 16 at 320 °C and 10 2 torr, leading to extrusion of the diene 18 (Ar D 4-MeOC6H4) to afford (C)-clavularin A 17 in 84% yield, is the final
O |
|
|
|
|
|
O |
O |
|
|
|
R1 |
|
|
|
R1 |
|
|
|
O |
|
R2 |
O |
R2 |
|
|
||
|
(1) |
|
(2) |
O |
H |
|
O |
|
H |
|
|
|
|
|
|
|
O |
|
|
|
H |
|
|
|
(3) |
|
(4) |
476 R. Alan Aitken and Andrew W. Thomas
step in its six-step synthesis from cyclohepta-2,6-dienone21. A C C triple bond may also be protected by Diels Alder reaction and norbornadiene acts in this sense as a masked form of acetylene. This has been exploited by Paton and coworkers to provide a synthesis of 3-vinylisoxazole22. Addition of the 1,3-dipole, acrylonitrile oxide, to norbornadiene gave 19 as a mixture of exo and endo isomers which lost cyclopentadiene to give the
product 20 in virtually quantitative yield upon FVP at 350 400 °C and 3 |
ð |
10 3 |
torr. |
||
|
|
|
|
|
|
O |
O |
R |
R |
R |
R |
|
•• |
N2 |
|
O |
O |
(5) |
|
O |
|
R |
|
R |
R |
|
R |
• |
|
O
(6)
R1
O
R2
(8) |
(9) |
R1
•• O
(7)
O
R2 O
O
(11) |
(10) |
10. Pyrolysis involving compounds with CDC, CDN and CDO double bonds 477
FVP, 400 °C
(12) |
(13) |
O |
OH |
O |
|
(14) |
|
(15) |
|
O |
O |
|
O |
Me |
O |
Me |
|
Me |
|
||
Ar |
|
|
|
Me |
|
Me |
|
(16) |
|
(17) |
|
|
|
Me |
Ar |
|
|
|
|
|
|
+ |
|
|
|
(18) |
|
N |
N |
|
|
O |
O |
|
|
(19) |
(20) |
|
|
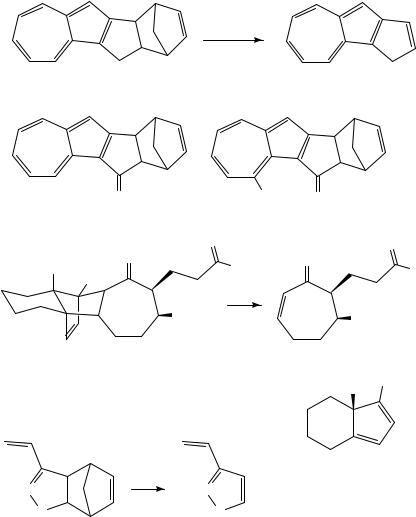
In some cases it is the diene component from the retro reaction which is the desired product and extrusion of a volatile alkene such as ethylene is then ideal. Pyrolysis of the 1,4-oxathiin systems 21 proceeds in this way to give the ˛-oxothiones 22 which for R D Pri, exists mainly as the enethiol tautomer 2323. Thermal extrusion of ethylene from 24 provides convenient access to the interesting fulvene 25 in quantitative yield24, and the corresponding reaction of 26 at 650 °C and 10 4 torr gives the cyclopentadienobenzopyrene 28 in 95% yield, presumably by way of the intermediate 2725.
A closely related reaction is the extrusion of the bridging atom from a norbornadiene system in the form of a carbene to leave a benzenoid product. This has been examined as a function of the substituents present for a range of examples 29 and it was found that when
478 |
|
R. Alan Aitken and Andrew W. Thomas |
|
|||
|
O |
|
O |
|
O |
|
|
|
|
|
(R = Pri) |
|
|
R |
S |
R |
S |
Me2 C |
SH |
|
(21) R = Me, Pri |
|
(22) |
|
(23) |
|
|
|
Me |
|
|
|
|
|
Me |
|
|
|
Me |
Me |
|
CO2 Me |
|
MeO2 C |
CO2 Me |
CO2 Me |
(25) |
(24) |
FVP
650 °C
(26) |
(27) |
(28)
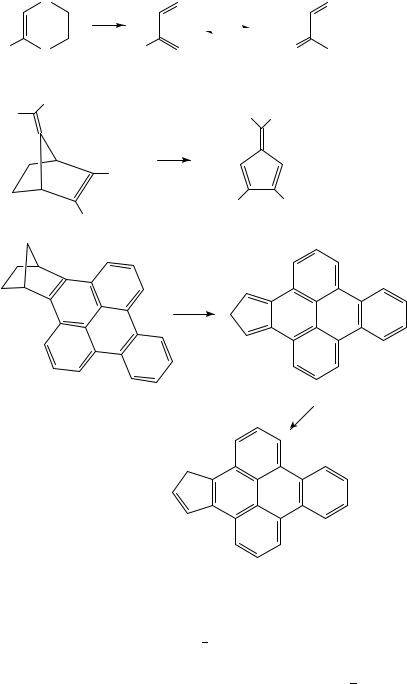
R1 and R2 are weak donors a radical mechanism dominates whereas, if they are strong donors, the carbenes 30 are formed by way of a zwitterionic intermediate26. In some cases such as 31 the fate of the carbene is uncertain and FVP at 600 °C gave dimethyl phthalate as the only isolable product27. Retro-Diels Alder reaction of the phosphole dimers 32 at 400 °C gives mainly the phospholes 33 in over 90% yield but a minor product is the dihydrophosphindole 34, suggesting at least some extrusion of the bridge in the form of the phosphinidene R1P:28. Finally, it should be noted that the retro-Diels Alder reaction
10. Pyrolysis involving compounds with CDC, CDN and CDO double bonds 479
|
|
|
|
|
|
|
O2 N |
|
|
|
R1 |
|
R2 |
|
|
|
|
|
|
|
|
|
|
+ |
R1 |
|
R2 |
|
|
|
|
CO2 Me |
(29) |
|
|
|
(30) |
|
(31) |
CO2 Me |
|||
|
|
|
|
|
|
|||||
|
|
R1 |
|
|
|
|
|
|
|
|
R |
2 |
P |
R |
3 |
R |
2 |
|
|
R2 |
R3 |
|
R2 |
|
|
R2 |
|
|||||
|
|
|
|
|
|
|
|
|||
|
|
R3 |
|
|
|
|
+ |
|
|
|
R3 |
|
|
|
|
P |
|
|
|
|
|
|
|
|
|
|
|
R |
3 |
|
P |
|
|
|
P |
|
|
R1 |
|
|
|
R1 |
|
|
|
R1 |
|
|
|
|
|
|
|
|
(32) R1−3 = H, Me |
|
|
(33) |
|
|
|
(34) |
|
||
X |
|
|
|
|
|
|
|
|
X |
|
|
|
|
+ |
+ |
|
O |
|
|
||
|
|
X |
SO2 |
|
|
|
||||
|
|
SO2 |
|
|
|
|
|
|
|
SO2 |
(35) X = CH2 , O |
|
|
|
|
|
|
(36) X = CH2 , O |
|||
X
O
(37)
usually depends on having an intact double bond between the atoms which were C(2) and C(3) of the diene component. Modifying this double bond, for example by epoxidation, is likely to result in a complete change in the pyrolysis behaviour as illustrated by the normal retro reaction observed for the tricyclic sulphones 35 at 675 °C, but the complete change for the epoxides 36 which give the divinylepoxides 37 at 700 °C29. An example in which a retro Diels Alder reaction similar to this still proceeds after modification of the double bond is described in Section IV.E.
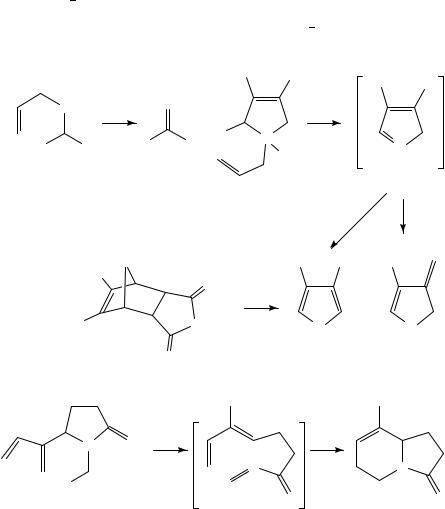
B. Retro-ene Reactions
Only a few examples of this useful thermal reaction have been described. The elusive thioformyl cyanide 39 has been generated by elimination of propene from 38 upon FVP at
480 |
R. Alan Aitken and Andrew W. Thomas |
700 °C and characterized by IR in an argon matrix30. Similar elimination of propene from 40 at 600 °C gives a mixture of 42 and 43 by isomerization of the initial product 4131. Interestingly, the silacyclopentadiene 42 can also be formed in a more conventional way by retro-Diels Alder reaction of 44 with elimination of maleic anhydride. The retro-ene reaction can also take place intramolecularly, and the intermediate 46 resulting from FVP of 45 at 800 °C undergoes an immediate intramolecular Diels Alder reaction to give the indolizidinone 47 as the final product32.
S
H CN
(38)
Me
Me
N
H
(45)
|
|
|
Me |
Me |
|
Me |
Me |
|
|
|
|
|
|
||
S |
|
|
|
|
|
|
|
H |
CN |
H |
Si |
|
|
|
|
|
|
|
Si |
|
|||
|
|
|
|
|
|||
|
|
|
|
H |
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
(39) |
|
|
(40) |
|
|
(41) |
|
H2 |
|
|
|
|
|
|
|
Si |
|
|
|
Me |
Me |
Me |
|
|
|
O |
|
|
|
|
|
|
O |
|
|
|
Si |
Si |
|
|
|
|
|
|
|
||
|
|
|
|
|
H2 |
H2 |
|
O |
|
|
|
|
|
|
|
(44) |
|
|
|
|
(42) |
(43) |
|
|
|
|
Me |
|
|
Me |
|
O |
|
|
|
|
|
|
|
|
|
|
N |
|
|
N |
|
|
|
|
|
O |
|
|
O |
|
|
|
(46) |
|
|
(47) |
|
C. Miscellaneous Reactions
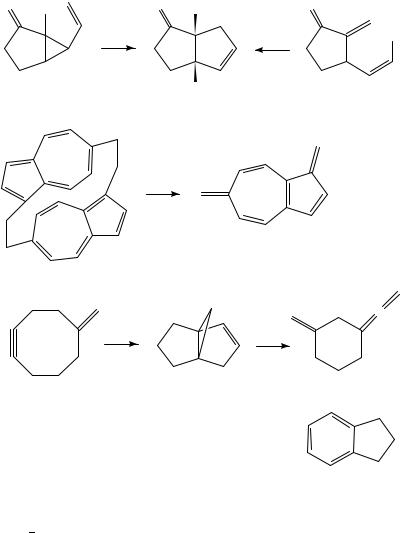
FVP of styrene at 850 °C gives benzene, ethylbenzene, naphthalene and other minor hydrocarbon products, while a mixture of isomeric methyl cyclohexa-1,3-dienes is converted under similar conditions mainly into benzene, toluene and ethylbenzene33. A vinylcyclopropane rearrangement occurs on FVP of 48 at 600 °C to give the cis fused cyclopentanone 49 and this product may also be prepared by FVP of the 1,4-diene 5034. The highly reactive 1,6-azulylene 52 can be generated by reversible ‘dedimerization’ of the
10. Pyrolysis involving compounds with CDC, CDN and CDO double bonds 481
cyclophane 51 and characterized by low-temperature UV-visible spectroscopy35. Several mechanisms have been discussed for the unusual conversion of the methylenecyclooctyne 53 into the propellane 54 on FVP at 450 °C, and this product reacts further at 650 °C to give 55 and indane36.
O |
Me |
O |
Me |
O |
|
|
|
Me
H
(48) |
(49) |
(50) |
(51) |
(52) |
•
(53) |
(54) |
(55) |
+
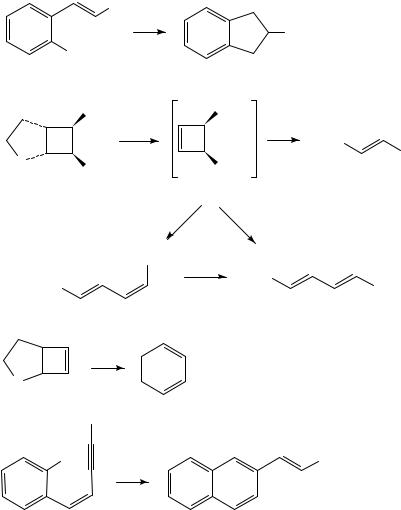
An important and frequently observed phenomenon in alkene pyrolysis is the ready equilibration of E and Z isomers at FVP temperatures above 500 °C. The apparently contrathermodynamic conversion of the E into the Z isomer has been quantified over the range 500 900 °C for stilbene, cinnamyl alcohol and cinnamonitrile37. In the last case, the proportion of Z isomer increases to 38% at 900 °C. In certain cases the diradical implicit in the isomerization process can be trapped by an intramolecular reaction and this is exemplified by the formation of 2-phenylindane in low yield from FVP of 56 at 700 °C37. The cis cyclobutene diester 58 is assumed to be formed as an intermediate in the FVP of the bicyclic sulphone 57 at 775 °C by loss of SO2 and ethylene. Under these conditions, however, it reacts further to give equal proportions of the E diesters 59
482 R. Alan Aitken and Andrew W. Thomas
and 60, by loss of acetylene and cycloreversion, respectively38. Independent pyrolysis of 58 gives the E, Z diester 61 at 500 °C but the E, E product 60 at 900 °C. The related cyclobutene 62 loses SO2 only to give cyclohexa-1,3-diene on FVP at 450 °C38. This presumably involves the intermediate bicyclo[2.2.0]hexene. We have also observed the E to Z isomerization phenomenon in the styrylalkynes formed from acyl ylide pyrolysis (see Section IV,D). When these are formed by FVP at 500 °C, the double bond is exclusively E as in the starting materials, but by 700 °C there has been up to 50% conversion to the Z isomer39. This proved to be important in the particular cases 63 with an ortho methyl group present, since at the much higher temperature of 900 °C these undergo cyclization to afford 2-alkenylnaphthalenes 6440.
|
Ph |
|
|
|
|
|
Ph |
Me |
|
|
|
(56) |
|
|
|
|
CO2 Me |
CO2 Me |
|
|
775 °C |
|
MeO2 C |
|
|
|
|
S |
|
|
CO2 Me |
CO2 Me |
CO2 Me |
|
|
O2 |
(59) |
||
(57) |
|
|
|
(58) |
|
||
|
|
||
|
500 °C |
|
|
|
CO2 Me |
°C |
|
|
900 |
|
|
MeO2 C |
|
MeO2 C |
CO2 Me |
|
|
||
|
(61) |
|
(60) |
S
O2
(62)
R1
Me |
R2 |
(63) |
(64) |
