

10. Pyrolysis involving compounds with CDC, CDN and CDO double bonds 493
Ph |
S |
PhN |
|
S |
|
|
|
|
|
|
|
||
|
|
• |
|
|
|
|
|
|
NH |
NH |
|
|
|
|
|
|
|
|
||
N |
|
|
|
PhN |
C C |
C O |
O |
N |
|
O |
N |
|
|
|
(149) |
|
(150) |
|
(151) |
|
|
|
Ph |
|
|
|
|
|
N |
|
N |
Ph |
N |
Ph |
|
N |
|
N |
|
N |
Ph |
|
H |
Ph |
|
|
|
|
|
|
|
|
|
|
|
|
(152) |
|
(153) |
|
(154) |
|
|
|
N |
|
N |
|
|
|
|
|
Ph |
|
|
|
|
|
N |
|
N |
|
|
|
|
H |
|
|
|
|
|
|
(155) |
|
(156) |
|
|
IV. COMPOUNDS CONTAINING C=O DOUBLE BONDS
The pyrolysis of carboxylic acids and their derivatives in the period up to 1980 was reviewed in a previous volume of this series84.
A. Aldehydes and Ketones
The characteristic thermal reaction of these compounds is decarbonylation and new examples include the formation of the ruthenocene compounds 158 by FVP of 157 at 640 °C85, and the decarbonylation of the cyclopentenone 159 to give the synthetically
R |
R |
|
|
|
|
R |
R |
R |
R |
|
|
R |
F |
R |
R |
F |
Ru |
|
R |
|
|
|
Ru F |
F |
O |
F |
F |
F |
|
F |
F |
F |
|
|
|
|
|
|
|
(157) |
R = H, Me |
|
(158) |

494 R. Alan Aitken and Andrew W. Thomas
useful 1,3-diene 160 in 72% yield upon FVP at 630 °C86. Remarkably, if the last reaction is conducted at 740°C, there is complete degradation to give a high yield of p-cresol. The transannular aldol reaction which occurs upon FVP of dione 161 at 500 °C to afford 162 is explained by the electrocyclic reaction of the functions as in 163 to give the enol 164, which is then activated towards attack on the other ketone function87. The alkoxycyclobutenones 165 are in equilibrium with their ring-opened vinylketene isomers 166 under FVP conditions above 400°C. At higher temperatures the alkoxy group migrates
to give the corresponding allenic esters 167 and for R D Et there is also some88 |
. |
||||||
of ethylene from the enol ether function of 166 to give the acetylketene 168 |
|||||||
|
|
|
|
|
|
|
Me |
|
Me |
O |
|
Me |
|
O |
|
|
|
|
|
|
|
||
O |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
O |
|
|
Me |
O |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|
Me |
(159) |
|
|
(160) |
|
|
(161) |
|
|
Me |
|
|
|
|
|
|
HO |
|
|
|
|
|
|
|
|
|
|
Me |
|
|
|
Me |
Me |
|
O |
|
|
O |
|
|
H |
|
|
|
H |
|
||
|
H |
|
|
|
|
||
O |
|
|
|
|
|
||
|
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(162) |
|
(163) |
|
|
|
(164) |
|
RO |
|
RO |
|
OR |
|
O |
|
|
|
|
|
|
|
|
|
|
|
• |
• |
O |
|
Me |
• |
O |
|
O |
|
|
|
|
O |
(165) R = Me, Et |
|
|
|
|
|
||
|
(166) |
|
(167) |
|
(168) |
||
elimination
B. Esters and Thioesters
The pyrolysis of benzyl benzoate has been examined in detail under FVP conditions over the range 750 900 °C89. While the main products are the expected diphenylmethane, toluene, biphenyl and bibenzyl, these are accompanied by a number of interesting minor products.
The thermal elimination of acetic acid from alkyl acetates bearing a ˇ-hydrogen atom, which proceeds by a six-membered ring transition state, has been widely used as a method

10. Pyrolysis involving compounds with CDC, CDN and CDO double bonds 495
of alkene preparation as exemplified by conversion of 169 into 170 by pyrolysis at 450°C in a nitrogen stream90, and the formation of the anti-Bredt alkene 172 from 171 at 400°C91. The method has also been used to form CDN double bonds in a few cases such as that of the highly reactive 2-azacyclopentadienone 174, formed by FVP of 173 at 350°C and trapped in a matrix92. By the use of suitable polyacetates, dienes and trienes can be obtained and Trahanovsky has described the generation of the interesting trienes 176 and 178 by FVP of the corresponding acetates 175 and 177 at 900 °C and 860 °C, respectively93.
Me |
|
Me |
|
|
|
|
Me |
|
|
Me |
|
|
OAc |
|
|
|
|
|
Me |
|
|
|
|
|
(169) |
|
(170) |
|
|
|
OAc |
|
|
H |
|
|
|
|
AcO |
|
|
|
|
|
N |
N |
|
|
|
|
|
||
|
|
|
|
O |
O |
|
(171) |
(172) |
|
(173) |
(174) |
|
|
|
AcO |
|
|
|
|
|
|
OAc |
|
AcO |
OAc |
|
|
|
|
|
|
|
|
OAc |
|
|
AcO |
|
|
|
|
|
(175) |
(176) |
|
(177) |
(178) |
Access to 1,3-dienes can also be gained by 1,4-elimination of acetic or benzoic acid from suitable ester substrates and this has been examined in detail for furan compounds by Trahanovsky and coworkers. Thus, the ‘furanoradialene’ 180 can be prepared in 13% yield by FVP of the diacetate 179 at 570 °C or in 30% yield from the corresponding dibenzoate at 610 °C94. These reactions are not as straightforward as they might appear since it is likely that migration of the acetoxy group to the 2-position by a [3,3] sigmatropic process followed by 1,2-elimination is involved rather than direct 1,4-elimination. While the benzoate 181 gives only 182, isolated in 51% yield as its dimer 183 upon FVP at 640 °C, the isomeric compound 184 gives both 183 and the cyclobutenone 187 under similar conditions95. This second product results from two sequential migrations of the benzoate group to give 185, which then suffers ˛-elimination of benzoic acid. The

496 |
R. Alan Aitken and Andrew W. Thomas |
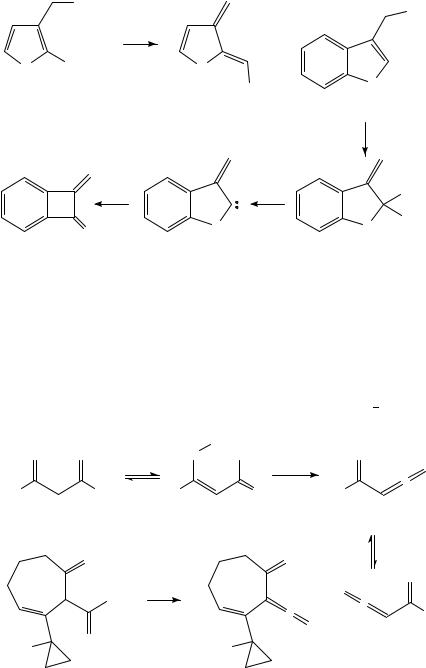
resulting allenylketene 186 finally cyclizes to give 187 in 21% yield. The benzofuran system 188 behaves in a similar way to 181, giving 189 upon FVP at 630 °C96, and the method has recently been extended to more hindered compounds such as 191 formed from the corresponding benzoate 19097. In the absence of an adjacent methyl group as in 192, the 1,3-migration still occurs to give 193 but this undergoes ˛-elimination to give the oxacarbene 194, which rearranges to provide the first synthesis of the methylenebenzocyclobutenone 19598.
AcO |
|
OAc |
|
|
|
Me |
O |
Me |
O |
|
|
|
|
|
|
||
|
(179) |
|
(180) |
|
|
|
OCOPh |
|
|
|
|
O |
Me |
|
O |
O |
O |
|
|
|
|||
(181) |
|
|
(182) |
|
(183) |
|
Me |
|
|
|
Me |
|
|
|
|
|
|
|
|
OCOPh |
H |
|
|
|
|
|
|
|
|
O |
|
|
PhC(O)O |
O |
|
|
|
|
|
||
|
|
|
|
|
|
(184) |
|
|
|
(185) |
|
|
|
|
Me |
|
Me |
|
|
|
|
|
|
O |
O |
|
|
(186) |
(187) |
OCOPh |
|
Me |
|
O |
O |
(188) |
(189) |
10. Pyrolysis involving compounds with CDC, CDN and CDO double bonds 497
|
OCOPh |
|
OCOPh |
|
|
|
|
O |
CH2 But |
O |
|
|
|
But |
O |
|
(190) |
(191) |
(192) |
|
|
|
H |
|
|
O |
OCOPh |
|
O |
O |
|
|
|
|
|
(195) |
|
(194) |
(193) |
The pyrolysis of ˇ-oxoesters results in elimination of an alcohol to give the corresponding ˛-oxoketenes. Thus methyl benzoylacetate 196, for example, loses methanol upon FVP at 700 °C to afford benzoylketene 197, which was shown by 13C labelling to
undergo degenerate rearrangement by means of a 1,3-phenyl shift99. A similar process occurs in the cyclic case 198, which affords the ketene 199 on FVP at 300 °C100. The ˛, -dioxoesters 200 also provide access to ˛-oxoketenes 201 with the loss of an alcohol and CO101.
The ˇ-enaminoesters such as 202 undergo similar loss of ethanol upon FVP and the
resulting iminoketene 203 cyclizes to afford 204102. Simple imidazole esters also undergo loss of an alcohol to give imidazole ketenes103. Thus FVP of 205 at 750 820 °C gives
|
|
|
H |
|
O |
O |
O |
OMe |
O |
|
|
|
− MeOH |
O |
Ph |
OMe |
Ph |
O |
• |
Ph |
||||
|
(196) |
|
|
(197) |
|
O |
|
O |
|
|
|
|
|
O |
|
OMe |
|
|
O |
|
|
• |
• |
|
|
|
|
Ph |
|
|
|
|
|
O |
X |
O |
|
X |
|
|
|
|
(198) |
(199) |

498 |
R. Alan Aitken and Andrew W. Thomas |
||
O |
O |
|
O |
|
|
|
O |
|
OR3 |
−R3 OH |
• |
R1 |
|
|
R1 |
|
−CO |
||
|
|
|
|
R2 |
O |
|
R2 |
(200) |
|
(201) |
|
206, while, under similar conditions, the 1-ester 207 first rearranges to the 2H isomer 208 which then gives 209, a product also accessible from 210.
|
NH |
OEt |
N |
|
N |
|
|
|
|
|
|
|
R |
O |
|
• |
O |
|
R |
O |
R |
||
|
|
|
|||
|
|
|
|
|
|
|
(202) |
|
|
(203) |
(204) |
|
N |
|
|
N |
N |
|
|
|
|
|
CO2 Me |
MeO2 C |
N |
• |
|
N |
N |
|
H |
O |
|
|
H |
|
|
|
|
|
|
|
(205) |
|
(206) |
|
(210) |
N |
N |
N |
|
|
H |
• O |
|
|
CO2 Me |
||
N |
N |
||
N |
|||
CO2 Me |
|
|
|
(207) |
(208) |
(209) |
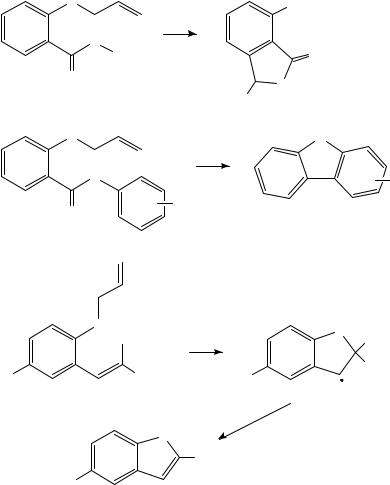
Pyrolysis of dialkyl oxalates is a well known method for the generation of alkyl radicals and FVP of 211 at 700 900 °C has been used to generate the o-allylbenzyl radical and examine its reactivity which is dominated by cyclization to give tetrahydronaphthalene 212104. The enol ester 213 gives the bicyclic ketone 215 on FVP at 420 °C by way of initial isomerization to the 1,3-diketone 214 whose enol form is ideally set up for an intramolecular Diels Alder reaction105. An intramolecular Diels Alder reaction is the first step in the remarkable pyrolytic conversion of the perfluoronaphthyl propiolate 216 into a mixture of the isomeric naphthocyclobutenones 217 and 218 and the naphthocyclopropene 219 upon FVP at 550 °C106. This is then followed by loss of CO and a series of 1,2- fluorine shifts to give the observed products. A number of useful synthetic procedures based on cyclization of ester-containing phenoxy radicals have been developed. Thus,

10. Pyrolysis involving compounds with CDC, CDN and CDO double bonds 499
O
O
2
(211) |
(212) |
O
O O
O
Me
Me
(213) |
(214) |
O O
Me
F |
F |
|
(215) |
F |
F |
|
|
||||
F |
F |
|
|
F |
|
|
|
O |
|
|
|
F |
O |
|
|
F |
|
F |
F |
|
H |
F |
O |
|
|
||||
|
|
|
|
|
F F |
|
(216) |
|
|
|
(217) |
|
F |
F |
|
F |
F |
|
F |
|
|
F |
|
|
+ |
|
|
+ |
|
|
F |
|
F |
F |
|
|
F |
|
F |
F |
F |
|
|
O |
|
|
F |
|
|
|
|
|
|
|
|
(218) |
|
|
(219) |
500 R. Alan Aitken and Andrew W. Thomas
the o-allyloxybenzoates 220 give the substituted phthalides 221 upon FVP at 650 °C in a process involving intramolecular abstraction of a hydrogen atom from the ester by Ož followed by cyclization107. The corresponding aryl esters 222 can similarly be used to gain access to substituted dibenzofurans and dibenzothiophenes 223 in a sequence involving initial ipso attack to give a spiro intermediate and loss of CO1082 . In the case of the cinnamates 224, the intermediates 225 from FVP at 650 °C undergo unusual homolytic loss of the ester group (as CO2 and R3ž ) to give the substituted benzofurans 226 in good yield109. This is to be contrasted with the behaviour of the corresponding phenols 227, which cyclize to the coumarins 228 with loss of R3OH on FVP at 750 °C. A surprising discrepancy in behaviour is observed for the two simple pyrrolylfumarates 229110. The dimethyl compound undergoes loss of methanol and cyclization upon FVP at 700 °C to afford 230, isolated as a mixture of stereoisomeric [2 C 2] dimers while, under the same
O |
OH |
O |
O |
CH2 R |
|
O |
O |
|
|
|
R |
(220)R = H, Me X
O
R
O
(222) X = O, S
O
R2
R1 |
CO2 R3 |
(224)
O
R2
R1
(221)
X
R
(223)
|
O |
R2 |
|
|
|
R1 |
|
CO2 R3 |
|
|
|
|
(225) |
|
(226)

10. Pyrolysis involving compounds with CDC, CDN and CDO double bonds 501
conditions, the diethyl ester fragments to give ethynylpyrrole 231. This is explained by twofold loss of ethylene to give the diacid, which then dehydrates to the anhydride, and this loses CO2 and CO to give the product. Complete loss of an ethyl ester group is also observed when acetylenic esters RC C CO2Et are pyrolysed at 750 °C111. The formation of both PhC CH and PhC CD from PhC C CO2CH2CD3 points to a combination of different mechanisms operating. FVP of the cyano silyl ester 232 provides an unusual route to trimethylsilylaniline 234 by migration of the silyl group from O to N with loss of CO2 to give 233, which then cyclizes112.
|
OH |
|
|
O |
O |
|
R2 |
|
|
|
|
R1 |
CO2 R3 |
|
R1 |
|
R2 |
|
(227) |
|
|
(228) |
|
|
|
|
CO2 Me |
|
|
CO2 R |
|
N |
|
|
|
|
R = Me |
|
|
|
|
|
|
|
(230) O |
H |
|
NH |
CO2 R |
|
|
|
|
|
R = Et |
|
|
|
|
(229) |
|
|
NH |
|
|
|
|
|
|
|
|
Me3 SiO |
|
(231) |
|
|
|
|
|
|
|
||
NC |
O |
|
|
|
|
|
• |
|
|
|
|
|
|
• |
|
|
|
|
|
|
|
|
|
|
|
|
NSiMe3 |
|
NHSiMe3 |
|
|
Me |
|
|
|
|
|
|
|
|
|
Me |
|
|
|
|
|
(232) |
|
(233) |
|
(234) |
|
C. Acid Chlorides and Amides
The characteristic pyrolytic process for acid chlorides is loss of HCl and this occurs for the bicyclic example 235 on FVP at 800 °C to give cyclohexa-1,2-diene 238 by way of the ketene 236 and carbene 237 as shown113. The product can be directly observed by low-temperature IR and forms a [2 C 2] dimer on warming up. FVP of o-toluoyl chloride 239 at 630 °C also results in loss of HCl to provide a dependable large-scale synthesis of benzocyclobutenone 240 in excellent yield114. Pyrolysis of trichloroacetyl chloride, Cl3C COCl, over a bed of zinc at 420 °C results in dechlorination

502 |
R. Alan Aitken and Andrew W. Thomas |
to generate dichloroketene, Cl2CDCDO, whose structure has been determined by electron diffraction115.
|
O |
|
|
• O |
: |
|
Cl |
|
(235) |
(236) |
(237) |
|
O |
|
|
O |
|
|
Cl |
|
|
Me |
|
(240) |
(239) |
(238) |
Amides of various types can act as a source of ketenes by pyrolytic elimination of an amine. Thus the acylpyrazole 241 undergoes loss of 3,5-dimethylpyrazole at 575 °C to give the alkylideneketene 242, which cyclizes to phenol in direct analogy to the imine 233 mentioned above116. This reaction has been extended for the formation of substituted phenols and naphthols by starting with suitably substituted analogues of 241. The cyanoacetylpyrazole 243 similarly loses dimethylpyrazole to give rise to cyanoketene upon FVP117. On the other hand, the pyrazolyl propiolate 244 undergoes a sigmatropic rearrangement to the alkylideneketene 245 on FVP at 650 °C and this then cyclizes to give the azaindole 246118.
The imidazole anilides 247 and 248 lose aniline upon FVP to give the ketenes 206 and 209, respectively103. The anilides 249 also behave like their oxygen analogues 220
O |
Me |
|
|
|
|
|
|
N |
|
• |
• |
N |
|
|
|
|
|
O |
|
|
Me |
Me |
OH |
|
|
|
|
Me |
|
(242) |
|
(241) |
|
|
O
Me
NC
N
N
Me
(243)
