

10. Pyrolysis involving compounds with CDC, CDN and CDO double bonds 483
A number of interesting pyrolytic methods involving CDC compounds with halogen present have been described. A range of halogenated cyclopropenes 65 undergo ring-opening upon pyrolysis with migration of one of the groups to give the isomeric allenes 6641. Pyrolysis of 1,2-dichloroethylene 67 in a stream of H2S gives the fused thienothiophenes 68 and 69 among other products42. The rather unstable ethynyl isocyanide 71 has been prepared by FVP of the dichlorovinyl isonitrile chromium complex 70 at 240 °C43.
X3 |
X4 |
|
||
|
|
|
X1 |
X3 |
|
|
|
X2 |
X4 |
|
|
|
||
X1 |
X2 |
|
||
(65) X1 − 4 = H, Cl, Br |
(66) |
|||
|
|
|
|
S |
Cl
Cl
(67)
H2 S |
+ |
S |
S |
S |
(68) |
|
(69) |
|
Cl |
||
(OC)5Cr C |
|
|
:C |
|
|
|
|
N |
|
|
N |
|
|
|
|
|
Cl |
||
(70) |
(71) |
||
In studies related to the stepwise synthesis of C60, Plater has developed pyrolytic dehydrochlorination methods for preparation of fused polycyclic aromatic hydrocarbons. The reactions, which are thought to involve electrocyclization followed by loss of HCl, require high FVP temperatures of 950 °C. Thus 72 and 74 are converted to the products 73 and 75 at this temperature44, while benzo[ghi]fluoranthene 77 is formed from 76 on FVP at 1030 °C45. At the even higher temperature of 1175 °C this product isomerizes to 78.
Cl
(72) |
(73) |

484 |
R. Alan Aitken and Andrew W. Thomas |
Cl
(74) |
(75) |
Cl
(76) |
(77) |
(78)
III.COMPOUNDS CONTAINING C=N DOUBLE BONDS
A.Imines, Oximes and Hydrazones
There have been relatively few recent studies in this area. FVP of the ˛-chloroimines 79 at 800 °C results in 1,5-loss of HCl to give the carbene 80 which cyclizes to afford the substituted indoles 81 in good yield46. The correspondingformamidines of the type
R |
|
R |
|
R |
|
Ar |
|
|
Ar |
|
|
|
|
|
|
|
|
|
N |
N |
Cl |
N |
Ar |
H |
(79) |
|
(80) |
|
(81) |

10. Pyrolysis involving compounds with CDC, CDN and CDO double bonds 485
82 undergo loss of dimethylamine under similar conditions, but this is accompanied by loss of Rž to give indole even in the case where R D Me. With the alkyl formamidines 83, FVP at 900 °C results in loss of Me2NH and both alkyl groups to give quinoline47.
FVP of simple oximes 84 proceeds mainly by loss of OHž and fragmentation of the resulting iminyl radicals to give PhCN, RCN and products derived from Phž and Rž . A minor process involves loss of only Hž from 84 and cyclization of the resulting radical to give the benzisoxazole 85, which rearranges to the benzoxazole 86 under the conditions used48. The benzyl oximes 87 similarly give ArCH2CN, RCN and products derived from Rž and ArCH2ž . FVP of ferrocenyl oximes in the range 650 680 °C has been described49. Fc CHDNOH gives Fc CN and Fc CHO while Fc CHDNOAc gives Fc CHO and acetic acid. The related oxime acetate Fc C(Et)DNOAc also gives Fc COEt. An interesting recent study involves the dimedone-derived oxime ethers 88 and 9350. FVP of the former at 450 500 °C gives a mixture of the isoxazole 89, the azirine 90 and the oxazole 91. As will be described in Section III.C, pyrolysis of isoxazoles is known to give oxazoles by way of the azirine, but the authors suggest that the initial loss of ethanol from 88 may give the vinylnitrene 92 rather than going directly to 89. FVP of the closely
related allyl ether 93 at 300 |
|
350 °C gives the three products corresponding to 89 |
|
91, but |
|||||
|
|
||||||||
in addition the nitrile 94. |
|
|
|
|
|
|
|
|
|
R |
|
|
|
R1 |
|
|
|
|
|
|
|
|
|
|
|
R2 |
|
|
|
H |
|
|
|
|
|
|
|
NOH |
|
N |
NMe2 |
N |
|
NMe2 |
Ph |
|
R |
||
(82) |
|
|
|
(83) |
|
|
(84) |
||
O |
|
|
|
O |
|
|
NOH |
|
|
N |
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
N |
|
Ar |
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
(85) |
|
|
|
(86) |
|
|
(87) |
|
|
FVP of the phenylhydrazone 95 of fluorenone at 600 |
|
1000 °C proceeds by loss of |
|||||||
|
|||||||||
PhNHž and ring-opening of the resulting iminyl radical to give 96, which cyclizes and abstracts a hydrogen atom to afford 9751. The N-aminoaziridine hydrazone acts as a useful thermal carbene source by loss of styrene and N2. This method has been applied to the ferrocene system 98 which, upon FVP at 380 °C, gives the carbene 99 which undergoes intramolecular insertion to afford ferrocenocyclobutene 10052.
B. Pyrazoles and Indazoles
The pyrolytic behaviour of simple pyrazoles has been studied in detail by Perez,´ Yranzo and coworkers. Not only have the products been identified, but in many cases

486 |
|
R. Alan Aitken and Andrew W. Thomas |
|
||
|
|
OEt |
N |
|
|
OH |
N |
O |
|
O |
|
|
|
|
N |
||
|
|
|
|
Ph |
|
|
|
|
|
|
|
|
|
Ph |
|
+ |
Ph |
Me |
O |
Me |
O |
Me |
O |
Me |
|
|
Me |
||
Me |
|
|
|
||
|
|
|
|
|
|
(88) |
|
(89) |
|
|
(90) |
|
|
|
|
|
Ph |
O
N
+
|
|
|
|
O |
O |
N: |
|
OH |
N |
|
|
Ph |
|
Prn |
Me |
O |
Me |
|
O |
Me |
|
|
Me |
|
(92) |
|
|
(93) |
|
|
NHPh |
|
CN |
|
N |
|
|
|
(95) |
|
(96) |
Me |
|
Me |
|
Ph |
|
N |
N |
|
|
|
|
Fe |
|
CH: |
|
Fe |
|
|
|
Me |
O |
|
Me |
|
(91) |
|
O |
|
CN |
|
Prn |
Me |
O |
Me |
|
|
(94) |
|
N |
(97)
Fe
(98) |
(99) |
(100) |

10. Pyrolysis involving compounds with CDC, CDN and CDO double bonds 487
rate constants have been determined which may distinguish between different possible mechanisms. FVP of 3,5-dimethylpyrazole 101 at 800 °C gives penta-1,3-diene and N532 . At the higher temperature of 900 °C, there is partial isomerization to penta-1,4-diene. The mechanism involves initial tautomerization to the 3H-form 102, which ring opens to the diazo compound 103. This is followed by loss of N2 to give the vinylcarbene 104 which undergoes a 1,4-H shift to afford the product. The parent compound behaves similarly to give propyne and N542 .
|
H |
|
H N |
|
|
|
Me |
N |
Me |
|
|
||
N |
N |
MeCH |
N2 |
|||
|
||||||
|
|
|||||
|
Me |
|
Me |
|
Me |
|
|
(101) |
|
(102) |
(103) |
|
H
Me
Me
(104)
A quite different process is observed for pyrazoles 105 which bear a ˇ-hydrogen atom containing alkyl group on nitrogen. ˇ-Elimination occurs by way of a five-membered transition state to give an alkene and pyrazole 106. This occurs on FVP of pyrazoles 107 with R D Et, Bun, Bus and But at 750 850 °C55,56, for R D CH2CH2Cl and CH2CH2Br at 600 670 °C57,58 and for R D CH2CH2Ph and CH(Me)Ph at 640 °C59. Theoretical support for the concerted mechanism has been obtained by MNDO methods60.
|
|
|
R |
|
N |
H |
N |
N |
|
N |
||||
N |
|
NH + |
||
(105) |
|
(106) |
(107) |
Other substituents on nitrogen give quite different results. FVP of N-phenylpyrazole at 700 °C causes ring-opening to PhNHCHDCHCN which is in equilibrium with PhNDCHCH2CN, while at 900 °C, N2 is lost to afford indene55. The phenyldimethylpyrazole 108 isomerizes to the imidazole 110 by way of the azirine 109 at 630 °C, while in this case reaction at 920 °C gives naphthalene55. FVP of N- benzoylpyrazole 111 at 600 720 °C proceeds as for 101 up to the stage of the carbene

488 |
R. Alan Aitken and Andrew W. Thomas |
112, but this can now undergo electrocyclization to afford 2-phenylfuran61. The products also include PhCONHCHDCHCN and its imine tautomer as for N-phenylpyrazole. FVP of 3,5-diphenylpyrazole at 600 800 °C involves initial tautomerization to the 3H-form which then directly loses N2 to give 1,3-diphenylcyclopropene, while under the same conditions 3-methyl-5-phenylpyrazole gives 1-phenylbutadiene and naphthalene62. Pyrolysis of both 3,5-bis(trifluoromethyl)pyrazole and the 3-methyl-5-trifluoromethyl analogue leads to complex mixtures of fluorinated alkynes, dienes and enynes at 640 700 °C63. In contrast to these results, bisand tris(pyrazolyl)methane undergo radical decomposition processes on FVP and, in the former case, pyrimidine is formed in low yield at 800 °C from the pyrazolylmethyl radicals produced64.
|
Ph |
|
|
NPh |
|
Ph |
|
Me |
N |
|
|
Me |
N |
Me |
|
|
|
|
|||||
N |
|
Me |
N |
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|
N |
|
|
Me |
|
|
|
Me |
|
|
|
(108) |
|
|
(109) |
|
(110) |
|
|
COPh |
|
|
|
|
|
|
|
N |
Ph |
O |
|
O |
|
|
|
N |
|
CH: |
Ph |
|
|
|
|
|
|
|
|
|||
(111) |
|
(112) |
|
|
|
|
|
The pyrolysis of indazoles 113 115 with bulky substituents R has also given some interesting results. For R D Ph3C, FVP of 113 at 400 °C leads to partial conversion into 114, while at 300 °C 114 gives 113. The less stable isomer 115 is converted at 300 °C into a mixture of 113 and 11465. For R D 1-adamantyl, 113 and 114 are again interconverted at 600 °C, while at 700 °C the new product 117 is formed from either by way of the azirine intermediate 11666.
|
|
|
R |
|
N |
NR |
N |
|
|
||
N |
|
|
|
|
N |
N |
|
R |
|
||
|
|
||
|
|
|
H |
(113) |
(114) |
|
(115) |
|
|
|
CN |
N |
|
|
|
NR |
|
|
NHR |
(116) |
|
(117) |
|
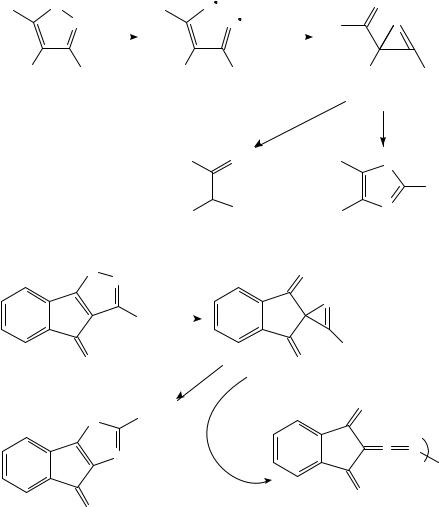
10.Pyrolysis involving compounds with CDC, CDN and CDO double bonds 489
C. Isoxazoles
The pyrolysis of isoxazoles has been examined in detail by Perez´ and they show some similarities to the behaviour of pyrazoles covered in the previous section. In general, FVP of 118 leads first to homolysis of the N O bond to give the diradical 119, which then rearranges to the acylazirine 120. If R3 D H, this rearranges to give the nitrile 122 as the final product, whereas in other cases it rearranges to the oxazole 121. Thus, 5- methyland 5-amino-4-methylisoxazole give the corresponding nitriles 122 at 550 °C and
400 °C respectively67, as does the 4-acetyl-5-methyl compound at 400 |
|
450 °C68. FVP |
|||||||||
|
|||||||||||
of 3,5-dimethyl- and 3-amino-5-methylisoxazole at 540 |
|
620 °C gives the corresponding |
|||||||||
|
|||||||||||
R1 |
O |
|
R1 |
O |
|
|
|
|
|
O |
|
|
|
|
|
|
|
N |
|||||
|
N |
|
|
|
N |
|
|
R |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R2 |
R3 |
|
|
R2 |
R3 |
|
|
|
R2 |
R3 |
|
|
|
|
|
|
|||||||
|
(118) |
(119) |
|
|
|
|
|
(120) |
|||
|
|
|
|
|
|
R3 = H |
|
|
|
||
|
|
|
|
R1 |
O |
|
|
R1 |
O |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R3 |
|
|
|
|
R2 |
CN |
|
|
R2 |
N |
||
|
|
|
|
|
(122) |
|
|
|
|
|
(121) |
|
O |
N |
|
|
O |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
N |
|
|
|
|
O |
But |
|
|
|
O |
But |
|
|||
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||
|
(123) |
|
|
|
|
(124) |
|
|
|
||
|
|
|
|
|
|
O |
|||||
|
O |
|
But |
+ |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
• N
N
2
O
O
(125)
(126)

490 R. Alan Aitken and Andrew W. Thomas
oxazoles 121, while for the trisubstituted 5-amino-3,4-dimethyl compound at 420 500 °C, the azirine 120 is the isolated product69. While 5-methyl-4-nitroisoxazole undergoes the expected reaction to afford the corresponding nitrile 122, the same product is also formed at 400 °C from the 3,5-dimethyl-4-nitro compound in a process involving anomalous loss of the 3-methyl group70. The indenone fused example 123 gives the azirine 124 and the unusual azine 125 at 300 400 °C, and when 124 is isolated and repyrolysed at 400 °C it gives mainly the oxazole 126, together with a small quantity of 12571. Support for the mechanism of 118 going first to the azirine 120 which can then either give 121 or 122 is provided by calculations using MNDO methods72.
D. Triazoles and Tetrazoles
The pyrolysis of 1-acyl-1,2,4-triazoles 127 proceeds readily under flash vacuum conditions at 800 °C to provide a useful synthesis of 5-substituted oxazoles 13073. The mechanism involves initial 1,2-acyl migration to give the 3H-form 128 which loses N2 to give the diradical 129 and this then cyclizes. For aromatic R groups yields are around 90%.
N |
N |
H |
N |
H |
N |
|
|||||
|
|
COR |
• |
• |
|
N N |
N |
N |
|
COR |
O |
|
COR |
|
|
|
R |
(127) |
(128) |
(129) |
(130) |
||
The 2,5-disubstituted tetrazoles 131 readily lose N2 upon pyrolysis to give the nitrile imines 132, which can be trapped by an added dipolarophile, detected spectroscopically, or in some cases isolated74. FVP of 131 (R D SiMe3) at 440 °C, for example, gives the nitrile imine which can be isolated as a solid at liquid nitrogen temperature and trapped by cycloaddition to methyl propiolate or bis(trimethylsilyl) fumarate75. In contrast to this behaviour, the 1H-tetrazoles 133, readily formed from cycloaddition of HN3 to nitriles, act as a useful source of carbenes by loss of N2 to give first 134 and then 135. This method
N |
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
− |
|||||||||
Ph |
N |
|
|
|
|
Ph |
|
C |
|
N |
|
N |
|
R |
|||||
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|||||||||||||
N |
R |
|
|
|
|
|
|
|
|
|
|
|
|
||||||
(131) |
|
|
|
|
|
|
(132) |
|
|
|
|
|
|
||||||
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
Ar |
|
|
CHN2 |
|
|
|
|
Ar |
|
CH: |
|||||
Ar |
N |
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(133) |
(134) |
|
|
|
|
|
(135) |
||||||||||||

10. Pyrolysis involving compounds with CDC, CDN and CDO double bonds 491
N
N
N
N
H
(136) |
(137) |
H
N
|
N |
|
H |
|
N |
|
N |
|
N |
|
N |
(138) |
(139) |
has been applied to the synthesis of the azulene derivatives 137 and 139 by pyrolysis of 136 and 138, respectively76.
E. Rings Containing Sulphur or Phosphorus
The 1,2,3,4-thiatriazoles 140 act as a convenient source of the highly reactive N2S. FVP results in fragmentation to afford ArCN and the dinitrogen sulphide, which can be detected by IR77. The spiro 1,2,5-oxathiazole 2-oxide 141 formed by addition of benzonitrile oxide to 9-sulphinylfluorene decomposes on solution thermolysis in benzene both by loss of benzonitrile to give the sulphene 142 and by loss of SO2 to give the nitrene 143. These then combine to give 144 as the final product78. Pyrolysis of the dioxathiazole S-oxides 145 results in loss of SO2 with concomitant migration of R to give the isocyanates, RNCO. This has been applied to the preparation of bis-isocyanates79 and trimethylsilyl isocyanate80.
The unusual oxazaphosphole ring system 146 undergoes loss of trimethyl phosphate upon FVP at 400 °C to give the azirines 147 for R D Ph and But while, at the higher temperature of 700 °C, the nitrile ylides 148 are formed81. Both 147 and 148 are readily hydrolysed to give RCONHCH(CF3)2.
F. Pyrimidines and Diazepines
The oxazole-fused pyrimidinethione 149 has been used as a source for generation of heterocumulene 151, an imine of carbon suboxide82. Under FVP conditions at 900°C, ring opening accompanied by a 1,2-phenyl shift first gives 150, which then loses HNCS and HCN to give the product that was characterized by IR in a matrix at 18 K.
The bicyclic dihydrodiazepines exemplified by 152 and 155 undergo a variety of pyrolytic processes83. In the 450 500°C range, FVP results in a series of 1,5-hydrogen shifts allowing interconversion of the cis and trans ring-fused compounds while, at 750 °C, more fundamental radical processes result in the formation of pyrazines, 152 giving 153 and 154 in yields of 46 and 4% respectively, and 155 giving 156 (50%).

N N
Ar N
S
(140)
N
R O
OS O
(145)
N
CF3
CF3
R
P(OMe)3
O
(146)
SO2
−PhCN
Ph
N
|
|
(142) |
S |
O |
|
|
|
|
O |
|
|
|
−SO2 |
Ph |
(141) |
|
|
|
|
|
|
|
N: |
|
|
(143) |
|
|
|
|
|
||
|
N |
+ |
|
− |
|||||
|
R |
|
R |
|
C |
|
N |
|
C(CF3 )2 |
|
|
|
|
|
|||||
|
|
|
|
|
|||||
−(MeO)3 PO |
|
|
|
|
|
|
|
|
|
|
CF3 |
|
|
|
|
|
|
|
|
|
F3 C |
|
|
|
|
|
|
|
|
(147) |
(148) |
|
|||||||
492
Ph
N
SO2
(144)
