
5. Chiroptical properties of compounds containing CDO groups |
225 |
OH
NHC(CH3 )3
O
O
+0.27 (286), +0.79 (267),
−6.77 (231)2 8 7
Chiral chalcone epoxides with oxygenation patterns of naturally occurring flavonoids and isoflavonoids were transformed into the corresponding ˛-hydroxydihydrochalcones similar to 115, and their CD was reported288. (C)-(˛R)-Enantiomers showed a weak negative 310 330 nm n ! Ł transition, followed by positive (260 290 nm) and negative (230 250 nm) ! Ł Cotton effects. Due to the profound influence of intramolecular hydrogen bonding (involving ˛- and 20-OH functions) on the conformation and hence the sign of the Cotton effect, conclusions based on CD regarding the absolute configuration of 115 analogues are reliable for similarly derivatized ˛- and 20 -OH functionalities (compare
114 and 115).
|
|
|
|
|
|
|
|
|
|
COPh |
|
|
|
|
|
N-CH3 |
H3 C-N |
|
|
||||
PhCO |
|
|
|
||||||||
|
|
O |
|
|
|
|
|
|
O |
||
|
|
|
|
|
|
|
|||||
|
|
|
|
OCH3 |
|
|
|
|
|
OCH3 |
|
|
|
|
|
|
|||||||
|
|
|
|
O |
|
|
|
|
|
|
O |
|
|
|
|
|
|||||||
|
|
|
|
O |
|
|
|
|
|
|
O |
(116) |
(117) |
||||||||||
−0.48 (330), +1.21 (277), +5.45 (253) |
−0.73 (344), −0.72 (263), +2.85 (240) |
||||||||||
The absolute configuration assignment of both chiral centers in 2-glycosyl-3- benzoylaziridines was achieved by comparing the CD properties of series cis- (116) and trans- (117) isomers289.
|
R |
R |
|
|
R |
R |
|
|
|
|
|
|
R = |
|
|
R |
R |
|
O |
|
|
|
|
|
−2.53 (325) |
−4.90 (334) |
−5.22 (327)2 9 0 |
|
|
|
R = CH2 OH (MeOH) +0.54 |
(326) |
||
|
O |
(C6 H12 ) |
−0.30 |
(366), −0.35 (348) |
|
|
|||
R |
|
|
−0.21 |
(334), +0.10 (326), |
|
|
+0.07 (315) |
||
|
|
|
||
|
R = CH2 OAc |
+0.55 |
(323) |
|
|
R = CH2 OGlu |
+0.57 |
(324)2 9 1 |
|
226 |
Stefan E. Boiadjiev and David A. Lightner |
|
|||||
|
|
|
O |
|
|
|
O |
RO |
|
|
|
RO |
|
|
|
|
|
|
OH |
|
|
|
OH |
R = H |
−5.21 (325) |
|
R = H |
−6.19 (330) |
|||
R = Glu −5.01 (325)2 9 1 |
|
R = Glu −5.56 (330)2 9 1 |
|||||
|
|
|
O |
|
|
|
|
HO |
|
|
R1 |
|
|
O |
Ph |
|
|
|
|
|
|
|
|
|
|
|
R2 |
|
|
|
|
|
|
|
OH |
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(−)-(S)-(118) |
|
|
R1 = CH2 OH, R2 = Me |
+5.54 (330) |
|
+2.24 (342), −4.77 (311), |
||||
R1 = Me, R2 = CH2 OH |
+5.60 (330)2 9 1 |
|
−3.02 (252), +4.43 (227)14 6 |
||||
H |
|
|
|
|
|
|
CH3 |
N |
|
Ph |
|
S |
Ph |
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
O |
|
O |
|
|
|
O |
|
|
|
(−)-(R)-(119) |
|
(−)-(R)-(120) |
|
|
|
||
−2.25 (368), +2.81 (326), |
−2.56 (364), +2.01 (322), |
+2.33 (346), |
|
||||
+0.99 (267), −5.93 (235)14 6 |
+2.57 (271), −11.32 (229)14 6 |
+0.61 (303), |
|
||||
|
|
|
|
|
|
−0.61 (286), +6.06 (255)2 9 2 |
|
The n ! Ł and ! Ł CD transitions of aza- (119) and thiaflavonone (120) were identified. Opposite signs were found for the Cotton effects of ( )-119 and ( )- 120 as compared to those of known ( )-(S)-118. According to the helicity rule, the heterocyclic ring must also adopt the opposite conformation because the phenyl substituent is equatorially oriented in all three flavonones. Thus, the (2R) absolute configuration was assigned to ( )-119 and ( )-120146.
H |
H |
|
|
|
|
|
|
N |
|
|
|
|
N |
|
H |
H |
O |
O |
|
CHO |
O |
|||
|
R CH2 CH3 |
|||
|
|
|
||
−1.43 (305), −1.02 (271) |
+0.49 (343), −0.11 (286), |
R = Me −2.40 (366), +1.75 (332), |
||
−1.02 (225), +9.30 (201)2 9 3 |
+1.42 (243), −4.30 (210)2 9 3 |
+0.60 (303), −1.95 (274) |
||
|
|
R = nPr +1.10 (362), −1.35 (332), |
||
|
|
−0.50 (304), +1.35 (273)2 9 4 |
||
5. Chiroptical properties of compounds containing CDO groups |
227 |
||
Et |
O |
O |
|
−6.18 (358), +4.97 (293), |
+6.29 (355), −1.17 (275), |
||
−13.5 (258), +38.2 (232)2 9 5 |
−0.63 (258), −2.61 (225)2 8 3 |
||
O |
O |
||
|
|
|
H |
|
|
|
H |
O |
O |
||
+20.2 (357), −11.5 (293), |
−7.12 (348), +7.29 (299), |
||
+58.6 (265), −48.2 (241)2 8 3 |
−16.8 (259), +18.9 (244), |
||
|
|
|
+56.8 (209), −54.8 (198)2 9 6 |
|
|
|
O |
H |
Ph |
||
|
|
|
|
H |
|
|
CO2 H |
|
|
|
|
|
O |
|
|
+0.50 (331), +1.2 (291), |
−1.06 (331), −0.86 (293), |
||
−3.9 (268), −7.2 (251), |
−0.35 (269), −6.12 (244), |
||
+38.1 (206)2 9 6 |
|
|
+6.66 (210)2 9 6 |
Octant diagram projections for the n ! Ł 335 nm transition of axially chiral conformational enantiomers ( G‡ D 19.8 kcal mol 1) of 2,6-dimethyl-1,5-bis(2,2- dimethylpropanoyl)naphthalene (121a and 121b)297 led to the assignment of absolute configuration.
O
O O
C |
C |
O O
(121a) |
O |
(121b) |
|
228 |
Stefan E. Boiadjiev and David A. Lightner |
The enantiomeric conformers 121a and 121b were resolved using low-temperature HPLC on a chiral stationary phase, and their differential absorption (A1 Ar) was reported as a signal from an on-line CD detector.
Two other ketones (122) containing axially chiral aromatic compounds were recently reported298.
R
H
(122)
R = COCH3 +0.14 (355), −26.3 (287)
R = COPh +0.03 (376), −33.6 (304)
4
COCH3
3 |
1 |
2
(+)-(123)
+0.10 (342), −0.19 (317), +28.3 (267), −54.7 (243), +26.2 (223), −22.0 (209)
2-Acetyl[a,c,e,g]cyclooctatetraene (123) has been resolved by repeated HPLC on swollen microcrystalline triacetylcellulose, and its CD has been reported299. Both longerwavelength Cotton effects were assigned to n ! Ł transitions from the acetyl group, possibly in two different conformations. The absolute configuration of (C)-123 was assigned as (R) for the 1-2 and 3-4 biphenyl units on the basis of calculations by the coupled oscillator technique of the rotational strengths of the dominant transitions (267 and 243 nm).
|
|
|
CH3 |
|
O |
R |
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
R1 |
R2 |
|
(−)-(S) |
|
|
|
|
R = H −11.12 |
(318) |
R1 |
= H2 , R2 = O +10.09 (321) |
|
R = OH −10.00 |
(321)3 0 0 |
R1 |
= O, R2 = H2 |
−9.19 (321)3 0 1 |
5. Chiroptical properties of compounds containing CDO groups |
229 |
O |
O |
|
O |
|
O |
||
−1.5 (475), −1.8 (405), +0.8 (358), |
−1.0 (475), −0.6 (400), −0.4 (370) |
||||||
−4.8 (316), +0.8 (290)3 0 2 |
|
+1.6 (320), −3.9 (270)3 0 2 |
|||||
|
O |
|
|
|
|
O |
|
O |
|
|
|
O |
|
|
|
|
|
O |
|
|
|
O |
|
MeO |
|
|
|
MeO |
|
O |
|
|
|
|
|
||||
|
CO2 H |
|
|
||||
|
|
|
|||||
|
|
|
|
|
|
||
|
|
|
|
|
|
|
O |
MeO |
|
|
|
MeO |
|
|
|
|
OMe |
|
|
|
|
OMe |
|
+1.32 (339), + 6.64 (297) |
|
−1.01 (338), −6.85 (304), |
|||||
−0.42 (275), +29.1 (243), |
|
+5.29 (276), −41.0 (244), |
|||||
−29.1 (221)3 0 3 |
|
+39.6 (218)3 0 3 |
|||||
|
|
O |
|
|
|
|
|
|
|
R |
|
|
|
|
|
|
R |
R1 |
|
|
|
|
R1 |
|
|
|
|
|
|||
|
CH3 |
COCH3 |
+2.45 (280), +10.79 (264), −11.83 (247) |
||||
|
CH3 |
C2 H5 |
+0.22 (284), +1.54 (260), +1.72 (254) |
||||
|
H |
CHO |
+2.45 (293), +11.63 (267), −12.85 (252) |
||||
|
OCH3 |
COCH3 |
|
+1.80 (287), +9.43 (257), −10.96 (240)3 0 4 |
|||
230 |
Stefan E. Boiadjiev and David A. Lightner |
|
|
R |
O |
H |
O |
|
|
||
|
|
|
|
O |
O |
|
|
R = H + 0.18 (327), −0.19 (287) |
+2.37 (311), −0.74 (267)2 6 0 ,2 6 1 |
R = OH −0.28 (326), +1.55 (264)2 6 0 ,2 6 1 |
|
HO
 O
O
O
O
−0.66 (330)3 0 5
O
O
O
OO
+2.8 (216)3 0 6
OR
OMe
O
OMe O
R = H +1.8 (406), +4.6 (344), −5.8 (307), +1.8 (283),
−11.8 (239), +13.6 (217)3 0 6 R = TBDMS +5.6 (344), −6.2(307),
+2.6 (283), −11.7 (240),
+16.5 (218)3 0 6
 OH
OH
O
O
|
O |
|
+ 0.92 (336)3 0 5 |
|
O |
OR1 |
|
|
O |
OR2 |
O |
R1 = R2 = H +2.8 (345), +6.4 (244), |
|
|
−4.5 (229)3 0 6 |
R1 = SO3 Na, R2 = H −4.8 (301), |
|
|
+3.3 (244), −11.5 (230)3 0 6 |
R1 = R2 = CH3 +1.8 (413), |
|
|
+1.4 (383), +1.7 (363), |
|
+2.8 (347), −5.5 (303), |
|
+4.6 (244), −8.9 (232)3 0 6 ,3 0 7 |
|
OH |
O |
H |
|
|
|
O |
O |
O |
−1.8 (335), +3.9 (250)3 0 8
|
5. Chiroptical properties of compounds containing CDO groups |
231 |
|||
|
OH |
|
|
|
|
OR |
H |
H |
O |
|
|
|
|
|
|
||
|
|
N |
|
|
|
|
O |
S |
|
|
O |
|
|
|
|
|
|
OR |
O |
O2 |
O |
O |
|
|
|
||||
R = Me +0.7 (397), −3.8 (326), |
|
+1.8 (399), −3.5 (337) |
|
|
|
|
+2.0 (281), +3.4 (235) |
|
+4.0 (304), −1.2 (277), |
|
|
R = H +0.5 (420), −4.5 (325), |
|
−6.6 (243)3 0 9 |
|
|
|
|
+2.1 (282)3 0 8 |
|
|
|
|
|
O |
|
|
|
|
|
O2 |
|
|
|
|
|
S |
|
|
|
|
|
N |
|
O |
|
|
|
|
|
|
|
|
|
H |
O |
|
|
|
|
O |
|
|
|
|
|
+2.0 (414), −4.0 (346), |
|
|
|
|
|
+2.8 (303), +6.3 (282), |
|
|
|
|
|
−4.0 (258), −6.7 (239)3 0 9 |
|
|
||
R1O |
H |
|
|
|
|
O |
H |
|
|
|
|
O |
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
R1 = β-D-Glu, R2 = H |
|
|
|
OR2 |
|
−4.01 (320), −1.27 (295), |
|
|
|
|
−4.03 (275), +5.45 (239) |
|
||
|
O |
|
|
||
|
OMe R1 = R2 = H |
|
|
||
|
|
|
|
||
|
OMe |
|
−4.79 (320), −1.30 (295), |
|
|
HO |
|
−5.09 (275), +7.88 (237)310 |
|
||
|
|
|
|
|
|
O |
O |
|
|
|
|
|
O |
|
|
|
|
−0.39 (320), −0.82 (295),
O |
−1.39 (275)3 10 |
|
OMe |
|
OMe |
232 |
Stefan E. Boiadjiev and David A. Lightner |
||||
|
|
|
|
|
Rifamycin S (R = OH) |
|
|
|
|
|
MeOH/H+ |
|
|
|
|
|
+7.06 (437), −4.89 (353), +12.4 (300), |
|
|
|
19 |
|
−24.6 (278), +41.4 (225)3 11 |
AcO |
|
|
|
MeOH/OH− |
|
OH |
OH |
1 |
|
||
|
+2.81 (515), +6.38 (426), +22.9 (338), |
||||
|
|
|
|||
|
R |
O |
H |
|
−22.7 (314), −9.83 (270), +25.7 (227)3 11 |
|
|
|
16 |
R = OCH3 |
|
CH3 O |
|
|
N |
||
|
|
|
|||
|
|
|
|
−2 (470), +5 (342), +28 (310), −10 (285), |
|
|
|
|
|
|
|
|
|
|
O |
|
−15 (254), +81 (233), −74 (209)3 12 |
|
O |
|
|
|
16,17,18,19 - Tetrahydrorifamycin |
|
|
|
|
+4.81 (420), −2.29 (346), +12.0 (301), |
|
|
|
O |
|
|
|
|
O |
|
|
−5.13 (274), +7.38 (239)3 11 |
|
|
|
|
|
||
O
Hexahydrorifamycin
+3.93 (433), +3.99 (333), +8.62 (300), −3.55 (274), +3.02 (252), +2.37 (235)3 11
The observed complex CD of the antibiotic rifamycin chromophore was simulated (250 190 nm) by means of coupled oscillator theory, from coupling of the long-axis polarized aromatic transition with dienone transition312.
O O
O
|
|
O |
O |
|
OCH3 |
|
|
|
|
|
−5.48 (354), −2.0 (264), |
|
|
||
|
|
|
−19.38 (219)3 13 |
|
|
|
|
OH |
O |
OH |
OH |
O |
OH |
CHO |
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
HO |
O |
|
|
|
|
|
|
|
|
|
O |
|
|
O |
|
|
H |
|
CO2 Me |
|
|
|
CO2 Me |
OH |
|
|
|
|
|
|
|||
|
+3.50 (370), +2.20 (340), +3.06 (330), |
|
−1.71 (405), +4.29 (360), |
||||
|
−2.63 (269), −2.85 (263), −12.7 (224)3 13 |
|
+2.15 (312), −6.15 (276), |
||||
|
|
|
|
|
|
+8.01 (237)3 13 |
|
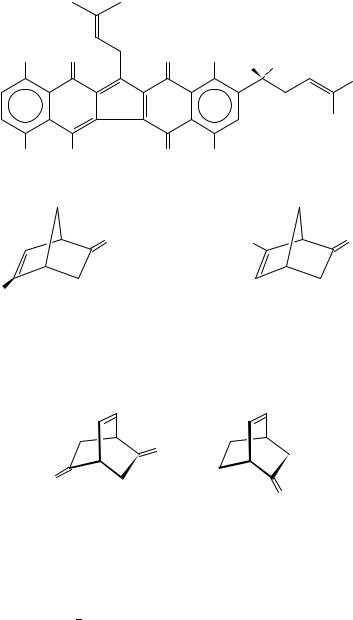
An octant projection diagram predicts a dissignate halogen contribution in the C 5 halogen-substituted bicyclo[2.2.1]hept-5-en-2-ones (125 and 126), and a consignate
5. Chiroptical properties of compounds containing CDO groups |
233 |
||||||
|
|
|
|
|
|
N |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
O |
|
OH |
H |
|
H |
|
|
|
|
|
|
|
||
|
|
O |
|
|
|
OH |
O |
|
|
|
|
|
|
|
|
O |
O |
OCH3 |
|
|
N |
|
|
|
|
|
|
|
H |
|
|
−2.58 (325), +1.42 (290), |
|
+1.8 (301), −4.56 (269)3 13 |
|||||
+1.42 (270)3 13 |
|
|
|
|
|
|
|
OH |
O |
|
|
OH |
|
O |
|
|
O |
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
HO |
R |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
|
OH |
|
O |
|
R = H −4.60 (299), −7.60 (260), |
|
+0.06 (330)3 16 |
|
||||
+1.40 (231), +6.16 (195)3 14 |
|
|
|
|
|
||
R = bond sh −2.63 (307), −17.93 (273), |
|
|
|
|
|||
|
+17.90 (229) 3 13 -3 15 |
|
|
|
|
|
|
|
OH |
O |
|
O |
|
OH |
|
|
|
|
H |
H |
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
H |
|
|
|
|
|
OH |
O |
|
O |
|
OH |
|
|
|
−0.08 (500), −0.08 (280)3 16 |
|
|
|
||
|
OH |
OH |
O |
OH |
H |
OH |
|
|
|
|
|
|
|
||
OH |
O |
O |
OH |
−0.55 (304), +0.55 (276), −1.27 (234)3 16
234 |
Stefan E. Boiadjiev and David A. Lightner |
OH |
O |
O |
OH |
H OH |
|
|
|
|
OH |
OH |
O |
OH |
|
−0.91 (302), −0.91 (270), +3.45 (234), +4.73 (218)3 16 |
||
|
|
|
|
O |
X |
O |
|
X |
|
|
|
|
|
(124) |
X = H |
+18.8 |
(306)2 0 2 |
(127) X = Cl +18.6 |
(320), +28.8 (308), |
|
(125) |
X = Cl |
+20.4 |
(318), |
+30.8 (306), |
+23.2 |
(297), −6.6 (232)2 0 3 |
|
|
+25.8 |
(295), −5.6 (220)2 0 3 |
(128) X = Br +23.4 |
(320), +34.0 (308), |
|
(126) |
X = Br |
+23.3 |
(320), |
+34.4 (308), |
+28.4 |
(297), −6.6 (232)2 0 3 |
+28.3 (297), −7.2 (220)2 0 3
|
O |
O |
|
|
O |
(−)-(129) |
(+)-(130) |
−16.9 (304) |
|
contribution in C 6 substituted enones 127 and 128. The experimental data showed no significant difference (n ! Ł ) between unsubstituted and ˇ- or -halogenated derivatives203. The results confirmed the interpretation that the n ! Ł transition in ˇ, -enones is to a large extent determined by an admixture of ! Ł olefinic transitions polarized along the Cˇ C direction202,317.
The C2 symmetrical 5,7-dioxobicyclo[2.2.2]oct-2-ene (129) CD has been reported96. Its absolute configuration was assigned utilizing the extended octant rule200 and correlated with that of (C)-130. Optically active 129 was reported earlier as formed in 3.5%ee by preferential photodestruction of (1S,4S)-enantiomer using right circularly polarized light318.
