
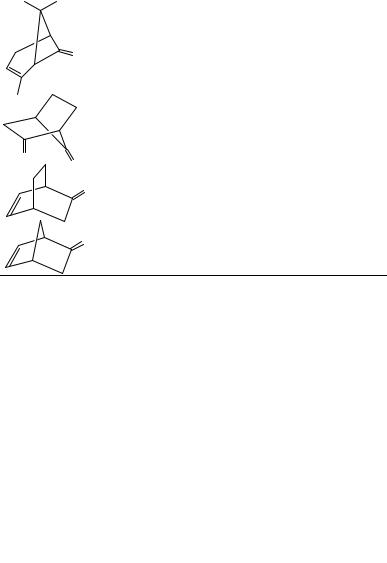
5. Chiroptical properties of compounds containing CDO groups |
205 |
||||||
TABLE 7. (continued) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ketone |
|
a |
cos |
Obs. ε |
a |
cos |
|
|
O |
75° |
C0.26 |
C4.71 |
76° |
C0.24 |
|
|
|
70° |
C0.34 |
C5.69 |
74° |
C0.28 |
|
CH2 |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
60° |
C0.50 |
C12.0 |
55° |
C0.57 |
|
|
O |
55° |
C0.57 |
C18.8 |
48° |
C0.67 |
|
|
|
||||||
aSee Figure 14.
FIGURE 14. Chirality rule reference frame for CDO (X, Y, Z) and CDC (X0 , Y0 , Z0 ) chromophores of ˇ, -unsaturated ketones. The C˛ atom connecting the chromophores lies in the XZ plane; the Y0 axis is perpendicular to the plane of the CDC group. denotes the angle between Z and Z0 axes. The chirality rule is given by: cos D sign XY Ð cos , where X and Y are the Cartesian coordinates of Cˇ andis the angle between the CDO axis and Er (the electric dipole moment associated with the transition). Applications are shown in Table 7
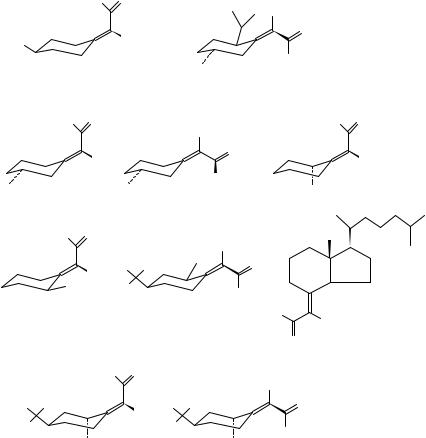
206 |
Stefan E. Boiadjiev and David A. Lightner |
transition, x and y are the Cartesian coordinates of Cˇ , and is the angle of intersection of axes drawn along and through the CDO and CDC bonds (Figure 14). The correlation for the limited number of examples of Table 7 is quite good, but only one example has been reported with a negative value of cos . Additional experimental support of the chirality rule has been published recently203, and a detailed theoretical treatment of 2- norbornenone supported the concept but not all of the assumptions, while clearly showing the importance of extrachromophoric perturbers204. Recently, a theoretical analysis was presented for ˇ, -unsaturated ketones based on coupling between ! Ł and n ! Ł transitions205.
V. COMPILATION OF UNSATURATED KETONE AND ALDEHYDE CD
Walborsky and coworkers206 have reported the CD of series conjugated cyclohexylidene aldehydes and ketones. These compounds served as additional examples supporting the planar diene rule for CD ! Ł transition208.
|
|
|
H |
O |
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
H |
|
O |
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
R = CH3 |
−0.61 (347), +2.08 (230) |
+0.44 (348), |
−2.70 (236)2 0 6 |
|
|||
R = C(CH3 )3 |
−0.72 (347), +2.65 (231)2 0 6 |
|
|
|
|||
|
H |
|
O |
|
|
H |
O |
|
|
|
|
|
H |
|
|
|
|
|
H |
|
O |
|
H |
|
|
|
|
|
|
||
|
|
|
|
|
H |
|
|
−0.70 (347), +0.82 (230) |
2 0 6 |
+0.62 (348), |
−4.55 (232)2 0 6 |
−0.87 (348), +4.93 (323)2 0 6 |
|||
|
|
|
|
|
|||
|
H |
|
O |
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
H |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
−0.65 (346), |
+3.58 (231)2 0 6 |
+0.58 (347), −3.36 (232)2 0 6 |
H |
|
|||
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
O |
|
|
|
|
H |
O |
|
+1.74 (347), |
−5.76 (236)2 0 7 |
|
|
|
|
|
|
||
|
|
|
|
|
|
H |
|
|
|
|
|
H |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
−0.80 (347), |
+3.48 (233)2 0 6 |
+0.47 (347), −2.16 (233)2 0 6 |
|
||||
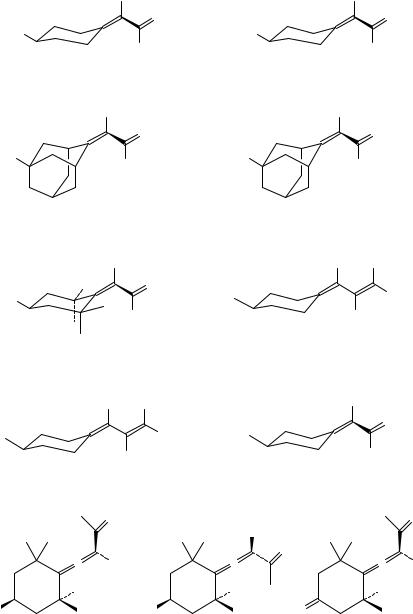
5. Chiroptical properties of compounds containing CDO groups |
207 |
|||
|
H |
|
H |
|
|
O |
|
O |
|
RO |
|
RO |
|
|
|
H |
|
CH3 |
|
R = H |
+0.29 (346), −1.00 (230) |
R = H |
−0.05 (337), +2.30 (238) |
|
R = TBDMS |
+0.57 (347), −3.30 (235)2 0 9 |
R = TBDMS |
−0.07 (345), +0.90 (240)2 0 9 |
|
|
H |
|
H |
|
|
O |
|
O |
|
R
|
|
H |
|
R = OH |
+0.78 (345), |
−4.2 |
(231) |
R = CH3 |
+0.13 (345), |
−1.9 (235)2 0 9 |
|
|
|
H |
|
|
|
|
O |
R |
|
|
|
|
|
H |
|
R = OH |
−1.09 (357), +16.39 |
(242) |
|
R = CH3 |
−0.94 (358), +12.45 |
(244)2 10 |
|
R
CH3
R = OH −0.12 (337), +4.30 (239)
R = CH3 −0.03 (332), −0.80 (240)2 0 9
H H
R
H
R = CHO −0.22 (350), +0.30 (277) R = COCH3 −0.04 (347), +1.20 (287) R = COtBu −0.03 (350), +1.60 (287)2 11
|
H |
R |
|
|
H |
|
|
|
|
|
|
O |
|
|
|
H |
|
|
|
|
|
|
H |
|
|
R |
|
R = CHO |
−0.40 (289) |
R = CH3 |
−0.05(363), +2.9 (235) |
|
||
R = COCH3 |
−1.40 (290)2 11 |
R = C(CH3 )3 |
−0.03 |
(367), +2.7(237)2 11 |
||
|
O |
|
|
|
|
O |
|
|
|
H |
|
|
|
|
|
|
O |
|
|
|
• |
H |
|
• |
|
• |
H |
OH |
|
OH |
|
OH |
|
|
AcO |
|
AcO |
|
O |
|
|
+0.06 (325), −5.4 (250), |
+0.3 (330), |
−4.1 (253), |
+2.3 |
(296), −6.6 (253), |
||
+6.6 (225) 2 12 |
|
+7.1 (227)2 12 ,2 13 |
+7.8 |
(229)2 12 |
|
|
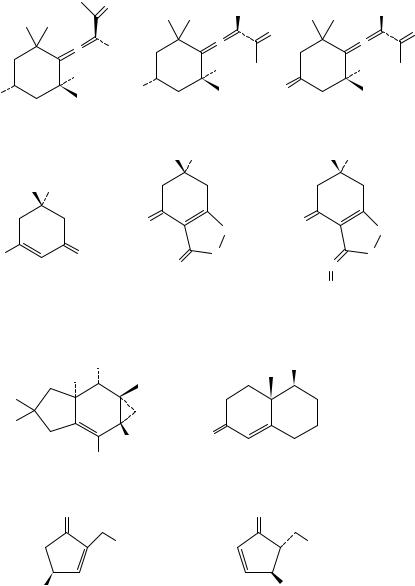
208 |
Stefan E. Boiadjiev and David A. Lightner |
|
|
|||
|
|
O |
H |
|
H |
|
|
|
|
|
|
||
|
|
|
• |
O |
• |
O |
|
• |
H |
|
|
||
|
|
|
|
|
||
|
OH |
|
OH |
|
OH |
|
|
|
|
|
|
|
|
AcO |
|
AcO |
|
O |
|
|
|
|
|
|
|
|
|
−0.3 (330), |
−1.5 (251), |
−0.3 (321), +0.2 (297), |
+2.2 (296), −3.0 (253), |
|
||
+2.9 (235)2 12 |
+5.0 (230)2 12 |
|
||||
+1.2 (231)2 12 |
|
|||||
|
|
|
|
|
||
|
|
Ph |
H |
Ph |
H |
|
Ph |
H |
|
|
|
|
|
|
|
O |
S |
O |
S |
|
|
|
|
|
|
||
MeS |
O |
S |
S |
S |
S |
|
|
|
|
|
|
||
|
|
|
|
O |
|
|
−1.73 (329), +2.55 (285), |
+0.26 (502), |
−0.76 (400), |
+0.96 (464), |
−0.71 (390), |
|
|
+5.00 (211)2 14 |
|
+2.83 (317), |
+5.48 (272), |
−1.04 (360), |
−1.40 (275), |
|
|
|
−3.75 (235)2 15 |
+1.48 (247)2 15 |
|
||
|
OH |
|
OAc |
|
|
|
|
H |
|
|
|
|
|
CHO O
CHO
+0.47 (367), +1.42 (338), −0.8 (322), +7.4(236)2 17 −10.17 (285), +5.45 (239)2 16
|
O |
|
O |
|
|
|
|
CO2 H |
|
|
CO2 R |
RO |
|
|
|
OH |
|
|
|
|
|
|
|
R = H |
+2.44 |
(318), |
R = H |
−2.35 (320), |
|
|
−16.46 (224) |
|
+18.54 (221) |
||
R = PhCO +1.89 |
(320), |
R = Me |
−1.41 |
(312), |
|
|
−28.86 (230)2 18 |
|
+9.30 |
(222)2 18 |
|
5. Chiroptical properties of compounds containing CDO groups |
209 |
The absolute configuration of a new sex pheromone (hepialone, 91) was determined219 on the basis of a rule for (nearly) planar ˛,ˇ-enones198. The (R) configuration of 91 is supported also by the negative n ! Ł CD Cotton effect of the saturated ketone 92 (obtained by ˇ-methylation of 91) where the equatorial ˇ0-ethyl group has larger contribution than equatorial ˇ-methyl group and ˇ-axial methyl group has small or dissignate contribution.
O
O H
(91)
+0.89 (312), +2.15 (261)2 19
O
Cl
|
|
|
CH2 H |
+ |
|
|
CO2 − |
H3 N |
CONH |
||
|
−0.45 |
(312), |
|
|
+7.03 |
(245)2 2 1,2 2 2 |
|
|
OH |
|
|
O |
|
|
OCH3 |
+3.09 (371), −3.70 (329), +1.58 (247), −1.06 (226)2 2 4
|
O |
|
|
|
|
O |
|
|
|
|
|
CH3 O |
|
|
|
|
|
|
|
|
|
|
O |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
(92) |
|
|
|
|
|
|
|
(−) (300)2 19 |
|
+2.88 (330), |
+1.52 (300), |
||||
|
|
|
+7.52 (270), |
−2.88 (220)2 2 0 |
|||
|
O |
|
|
|
|
O |
|
|
|
|
|
O |
|
|
O |
|
O |
|
|
|
|
O |
|
+0.49 (359), |
+1.03 (344), |
−0.42 (360), |
− 0.87 (344), |
||||
−0.23 (285), |
+0.69 (260)2 2 3 |
−13.0 (287), |
+9.1 (260)2 2 3 |
||||
|
|
|
R2 |
|
R1 |
|
|
|
|
|
|
|
|
|
|
|
Ve |
|
O |
|
O |
|
|
|
|
|
R2 |
|
|
|
|
|
R1 |
R2 |
|
|
Ve = veratryl |
|
|
|
OMe |
H |
|
+1.68 (301), −3.47 (267), |
|||
|
|
|
|
−4.21 |
(258) |
|
|
HH +2.23 (301), −3.53 (267), −4.18 (258)
OMe bond +2.71 (301)2 2 5
210 |
|
Stefan E. Boiadjiev and David A. Lightner |
|
||
|
|
|
H |
|
|
|
|
|
O |
H |
|
|
|
|
H |
O |
N |
|
|
OMe |
|
||
|
|
|
|
|
|
|
|
|
|
N |
|
Pi |
O |
O |
O |
|
H |
|
|
||||
|
|
|
O |
|
|
|
Pi = piperonyl |
+0.69 (308), −0.46 (257), |
−4.5 (337), |
+1.1 (298) |
|
+4.12 (334), |
+18.03 (298), |
−0.58 (238)2 2 6 |
Perchlorate |
|
|
−3.61 (277), |
−6.18 (258)2 2 5 |
|
−4.6 (327), |
+2.5 (294)2 2 7 |
|
O |
|
H |
O |
|
|
|
N |
N |
|
||
|
|
|
|
||
|
|
N |
N |
|
|
|
|
|
|
H |
|
−6.3 (335), +1.0 (290) Perchlorate
−7.8 (328), +1.4 (290)2 2 7
O
(CH2 )n
N
HO
 H
H
OH
n = 1 +3.33 (335) n = 2 +3.19 (336)
n = 3 +2.71 (337)2 2 8
R
N
−2.6 (261)2 2 7
R
R = |
−0.49 (325)2 2 9 |
|
O |
|
OH |
R = |
−0.32 (326)2 2 9 |
|
O |
|
R |
|
N |
|
OAc |
HO |
|
|
HO |
|
|
|
|
O |
|
|
|
|
|
R = H,H |
−1.74 (292), |
+3.55 |
(236) |
R = H,H |
+1.96 (239), |
−0.56 (212) |
R = O |
−0.11 (385), |
sh −1.83 (291), |
R = O |
−0.21 (408), |
−0.61 (280), |
|
|
−2.32 (285), |
−1.97 |
(220)2 3 0 |
|
sh +3.09 (215), +4.01 (200)2 3 0 |
|
5. Chiroptical properties of compounds containing CDO groups |
211 |
A series of steroidal and related cisoid ˛,ˇ-enones was synthesized, and their CD was investigated231,232, adding new examples of enones containing -transoid or -cisoid allylic substituents. The relation between structure and observed Cotton effects was discussed in terms of previously published rules.
O |
|
|
|
|
|
||
(93) |
O |
||||||
|
(94) |
||||||
−0.10 (352), |
+0.23 (301), |
−0.55 (321), −4.01 (234) |
|||||
−3.25 (235), |
+5.30 (204) |
|
|
|
C8 H17 |
||
|
|
|
|
|
|
|
|
|
|
|
AcO |
|
|
|
|
|
|
|
AcO |
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
O |
||||
(95) |
|
|
|
(96) |
|||
+0.73 (323), −2.23 (226) |
−1.67 (320), |
−6.19 (232), |
|||||
|
|
|
|
+11.0 (198) |
|
|
|
|
|
|
C8 H17 |
|
|
|
C8 H17 |
AcO |
|
|
|
|
AcO |
||
|
|
|
|
|
|
|
|
AcO |
AcO |
|
|
|
O |
||
O |
|
|
|
|
|
||
(97) |
|
|
|
|
(98) |
||
−2.07 (325), +11.30 (225), |
−1.05 (332), −3.24 (248), |
||||||
+11.1 (200) |
|
|
|
+1.50 |
(218), +11.30 (193) |
||
All cisoid ˛,ˇ-enones studied, including compounds 93 98, obey the helicity rule for n ! Ł transition Cotton effects49,233,234. The sign of the 222 272 nm ! Ł band is also in accord with the helicity rule for unsubstituted enones, while the polar C O
212 |
Stefan E. Boiadjiev and David A. Lightner |
allylic bond strongly influences the ! Ł band235. However, compounds 93 and 94 have opposite signs to those predicted by the helicity rules. Substituents at the -cisoid position (as in 98) also show a remarkable contribution to the first ! Ł transition. The shorter-wavelength ! Ł transition (200 220 nm) exhibited a sign opposite to that of the n ! Ł transition231.
|
|
|
|
O |
|
|
|
|
R2 |
|
OH |
|
H |
|
|
|
O |
O |
H O |
O |
OH |
O |
O |
|
|
|
H |
|
|||
O |
|
O |
|
O |
|
|
|
|
R1 |
|
|
|
|
|
|
OH |
O |
OH |
O |
|
OH O |
|
|
|
|
|
|
|
|
|
|
|
R1 = OH, R2 = OH |
|
−1.76 (345), −0.23 (290) |
|
−1.90 (337), +4.00 (253)2 3 6 |
|
|
−1.96 (337), −1.70 (216) |
|
−1.95 (239)2 3 6 |
|
|
|
|
|
R1 = OH, R2 = H −2.04 (338), +0.30 (240), −1.53 (215)
R1 = H, R2 = OH −2.39 (338), +0.29 (241), −1.56 (209)2 3 6
Beecham and Collins237 analyzed the CD of 22 steroidal 4-en-3-ones. Based on similarities of Cotton effect amplitudes, ratios of heights of 0-0/0-2 lines and X-ray structural data, they concluded that the chromophore conformation is identical within the series possessing 17˛-, 17ˇ- and 6˛-substituents. A low-intensity, spin-forbidden singlet triplet n ! Ł transition might be the origin of a weak positive Cotton effect at 384 nm ( ε C0.001 to C0.020) observed in addition to the main vibronically-structured negative Cotton effect ( ε 1.20 to 1.50) for 0-2 line at 339 nm. In three of the 6ˇ-substituted compounds, this pattern was modified by a positive CD with the same vibrational progression, thus giving an overall bisignate shape: e.g. ε339 C0.51, ε297 0.13 for 6ˇ-methylcholest-4-en-3-one237.
S |
S |
|
S |
|
|
|
|
|
|
S |
S |
|
S |
|
O |
|
O |
|
|
|
HO |
O |
||
|
|
|
||
O |
|
|
Cl |
|
O |
O |
|
|
|
|
|
|
|
|
R1 |
|
|
|
|
R1 = H +0.20 (375), −4.83 (317), |
−1.73 (347), +2.13 (317), |
+1.03 (349), −3.95 (316), |
|
|
−3.64 (307), +2.74 (282)2 3 8 ,2 3 9 |
+2.13 (308), +11.0 (219)2 3 8 ,2 3 9 |
−3.54 (306), +7.12 (257)2 4 0 |
|
|
R1 = CH3 +0.22 (376), −4.45 (318), |
|
|
|
|
−3.33 (307), +2.46 (282)2 3 8 |
|
|
|
|
5. Chiroptical properties of compounds containing CDO groups |
213 |
O
OH
O |
|
|
O |
|
|
R1 |
SO2 Ph |
|
|
H SO2 Me |
|
|
R2 |
|
|
CO2 Me |
|
R1 = CO2 Me, R2 = H |
|
+0.52 (360), |
17β− |
|
|
+0.15 (378), −0.45 (326), −3.81 (240), +18.2 (219) |
|
+2.86 (294), +12.5 (220) |
|
||
|
|
17α− |
|
||
R1 = H, R2 = CO2 Me |
|
|
|
||
|
+0.53 (359), |
−1.99 (292), +4.5 (223)2 4 1 |
|||
+0.59 (360), −0.74 (265), −2.08 (238), +13.4 (220)2 4 1 |
|
||||
|
|
|
|
||
O |
OR |
O |
OH |
O |
OAc |
|
|||||
O |
O |
S |
CO2 H |
O |
|
||||
R= H |
−1.27 (324), +4.4 (286), |
−0.49 (324), +3.7 (286), |
|
+6.0 (342)2 4 2 |
R= Ac |
+8.8 (232) |
+2.3 (234)2 4 2 |
|
|
−1.16 (324), +4.1 (286), |
|
|
|
|
|
+11.1 (233)2 4 2 |
|
|
|
|
|
R2 |
|
R2 |
O CH2 OR |
|
|
|
OH |
||
|
|
|
|
|
O |
|
|
|
|
H |
|
|
|
|
|
O |
|
O |
H |
|
O |
|
|
|
|
|
|
|
|
|
R1 |
|
|
|
|
|
|
R2 = CH(CH3 )CH2 CH2 CO2 CH3 |
|
|
|
R1 |
= H |
+18.5 (231) |
+8.7 (224)14 5 |
R= H |
−4.71 (343), +11.41 (278), |
R1 |
= CH3 |
+17.4 (236) |
|
R= Ac |
−11.11 (226) |
R1 |
= (CH3 )2 |
+13.1 (232)14 5 |
|
−5.21 (343), +12.22 (277), |
|
|
|
−11.71 (225)2 4 3 |
|||
|
|
|
|
|
|
Caution in using a solid state conformation to explain CD data in solution was indicated in the work of Kirk and colleagues224. They found that the CD of 17˛-acetoxy-6˛- methyl progesterone (99) in solution did not deviate from that of analogous compounds, while the CD of crystalline samples of 99 in SE30 (silicone polymer) or KBr showed an inverted sign of the n ! Ł band. Exhaustive NOE experiments supported the CD data, concluding that the A ring of 99 in solution is in a normal (1˛,2ˇ) half-chair conformation while X-ray studies had shown that 99 crystallizes with an A-ring inverted (1ˇ,2˛) conformation.
214 |
Stefan E. Boiadjiev and David A. Lightner |
|
|
|||||||
|
|
O |
|
|
|
|
|
|
|
|
|
20 |
R |
|
Enone |
|
20-Oxo |
|
|
|
|
|
17 |
|
|
|
|
|
||||
|
|
n → π |
n → π |
π → π |
|
π → π |
||||
|
|
|
|
|
||||||
|
(99) R= OAc |
MeOH |
−1.2 (326) |
+3.4 (284) |
+6.4 (235sh) |
+8.6 (213) |
||||
O |
|
|
SE30 |
(+) (330) |
(++) (300) |
(+++) (255) |
|
(+) (205) |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
R= H |
MeOH |
−1.4 (330) |
+2.6 (292) |
+6.8 (240sh) |
|
+9.9 (220) |
||
|
|
|
KBr |
(−) (332) |
(+) (296) |
(++) (249) |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
20 |
R |
|
|
|
|
|
|
|
|
|
17 |
|
|
|
|
|
|
|
|
|
|
|
R= H |
MeOH |
−1.2 (325) |
+4.0 (284) |
+8.0 (233sh) |
|
+10.3 (214) |
||
O |
Progesterone |
SE30 |
(−) (328) |
(++) (298) |
(+++) (230) |
|
(++) (210) |
|||
|
|
|
|
|
|
|
||||
|
|
R= OH |
MeOH |
−1.0 (331) |
+2.6 (293) |
+6.7 (235sh) |
|
+10.3 (218) |
||
|
|
|
SE30 |
(+) (328) |
(+) (282sh) |
(++) (268) |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
R= OAc |
MeOH |
−1.7 (321) |
+3.3 (287) |
+4.6 (245sh) |
|
+9.4 (215) |
||
|
|
|
SE30 |
(+) (320) |
(+) (300sh) |
(++) (250) |
|
(−) (210) |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
C8 H17 |
|
|
|
|
C8 H17 |
|
|
C8 H17 |
AcO |
|
|
|
AcO |
|
|
|
AcO |
|
|
H |
H |
|
|
H |
H |
|
|
H |
H |
|
AcO |
O |
|
AcO |
|
|
O |
O |
|
|
O |
−0.79 (340), +16.4 (239)2 4 5 |
−0.89 (341), +18.8 (238)2 4 5 |
|
sh −0.98 (337), −3.58 (293), |
|||||||
|
|
O |
|
|
|
|
|
+12.2 (248)2 4 5 |
|
|
|
|
|
|
|
R1 |
R2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
CO2 CH3 |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
O |
|
|
|
|
|
|
|
|
OH |
H |
|
|
|
|
O |
|
|
H |
|
|
O |
|
|
|
|
|
|
||
O |
|
|
|
|
|
|
|
|
3 |
|
|
O |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
|
OH |
|
|
|
|
|
R1 = CH3 , R2 = OH |
|
|
|
−1.12 (317), −6.33 (275), |
|
|
|
+3.3 (376), −3.4 (320) |
|
|
||||
|
|
|
|
R1 = OH, R2 = CH3 |
|
|
||||
−2.79 (221)2 4 6 |
O |
|
|
−3.9 (376), +4.7 (320)2 4 7 |
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
R1 |
|
|
|
|
|
|
|
|
|
|
R2 |
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
H |
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
R1 = CH3 , R2 |
= OH |
|
|
|
|
||
|
|
|
−10.7 (349), +15.6 (304) |
|
|
|
|
|
||
|
|
|
R1 = OH, R2 |
= CH3 |
|
|
|
|
||
|
|
|
+9.1 (349), −12.2 (304)2 4 7 |
|
|
|
|
|
||
