

5. Chiroptical properties of compounds containing CDO groups |
175 |
TABLE 5. Chair conformers of 2-oxo-p-menthanol, octant projection diagrams, predicted and observed n ! Ł Cotton effects
Methanol interferes with intramolecular hydrogen bonding, and the equatorial isopropyl isomer is the major species present.
III.COMPILATION OF KETONE AND ALDEHYDE CD
A.Acyclic Ketones and Aldehydes
The acyclic aldehydes and ketones are expected to give substantially weaker CD than that of their cyclic counterparts because of the conformational mobility of the former, as was borne out by Djerassi and Geller75 in an early study of a series of optically active methyl-substituted aldehydes and ketones. Since then only a few studies on acyclic aldehydes and ketones have appeared76,77.
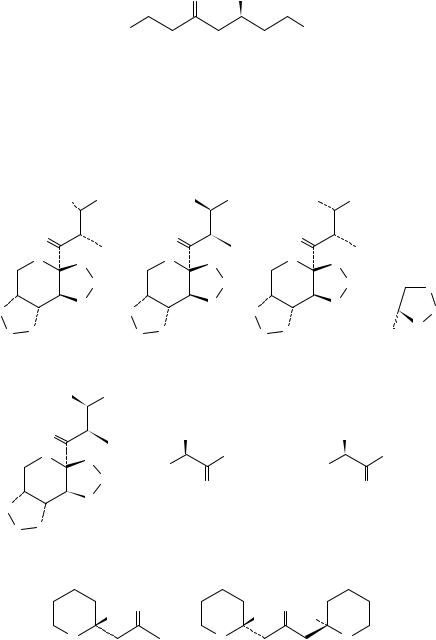
Potapov and coworkers78 applied the octant rule to (C)-2-s-butyl alkyl ketones (1), which exhibit very strong temperatureand solvent-dependent CD Cotton effects. The shift in ε from positive to negative by changing the steric demand of R group was rationalized on the basis of changing the conformational preference of the s-Bu group in 1.
|
COR |
|
O |
OH |
|||
H |
|
|
|
C2 H5 |
|
|
|
|
|
|
|
|
|
||
|
CH3 |
|
|
|
|||
|
(1) |
|
|
(2) |
|||
in EtOH: |
CCl4 |
−0.03 (310), +0.05 (277) |
|||||
R = Me, +0.076 (278); Et, (+) CD; |
CH3 CN |
−0.20 (283) |
|||||
i-Pr, (−) CD; i-Bu, +0.088 (280); |
|
|
|
||||
cyclo-C6 H11, +0.010 (310), −0.008 (275)
176 |
Stefan E. Boiadjiev and David A. Lightner |
|
|
O |
OH |
|
Me |
Me |
(3)
CCl4 −0.02 (312), +0.17 (280)
CH3 CN −0.18 (287)
The strong solvent dependence of the molecular rotation and CD of 2 and 3 on solvent polarity was attributed to formation/breaking of an intramolecular hydrogen bond between the carbonyl and hydroxy group79.
|
HO |
Ph |
HO |
Ph |
HO |
|
R1 |
|
|
O |
|
O |
|
O |
|
|
R = CHMe2 |
|
O |
O |
O |
O |
O |
O |
|
|
|
|
|
|
|
|
|||
|
|
R |
|
R |
|
|
R |
O |
|
|
|
|
|
|
|
|
|
|
|
O |
|
O |
|
O |
|
R1 = |
O |
|
O |
|
|
R |
|||
|
|
O |
|
|
|
|||
R |
O |
|
R O |
R |
O |
|
|
O |
|
|
|
H |
|||||
R = CHMe2 |
|
|
|
|
|
|
|
|
−0.76 (315)8 0 |
|
+2.70 (300)8 0 |
|
+0.14 (320)8 0 |
|
|
||
|
HO |
R1 |
|
|
|
|
|
|
|
O |
|
R |
|
|
|
R |
|
|
O |
O |
Me2 N |
n-Bu |
+ |
|
|
n-Bu |
|
|
R |
|
Me3 N |
|
|
|
|
|
|
|
O |
|
|
O |
||
|
|
|
|
|
|
|||
O |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
O |
|
|
|
|
|
|
|
+1.21 (297)8 0 |
|
−0.05(315), |
+0.06(281) |
R = Me |
|
|
+1.15(292) |
|
|
|
|
−2.53(318), +2.76(240) |
R = CHMe2 |
+1.67(295) |
|||
|
|
|
−1.09(304), +0.96(238)8 1 |
R = CH2 CHMe2 |
+1.36(295)8 1 |
|||
|
|
|
O |
|
O |
|
|
|
|
|
H |
|
H |
H |
|
|
|
|
N |
|
|
N |
|
|
N |
|
|
H |
|
|
H |
2HCl |
|
H |
|
|
|
−0.038(285) |
|
|
|
|
||
|
|
+0.084(287)8 2 |
|
|
|
|||
Sulfate −0.111(281)8 2
5. Chiroptical properties of compounds containing CDO groups |
177 |
Biologically active cyclic tetrapeptides (4 7) showed: (1) a conformational preference in the epoxyketone moiety; (2) no n ! Ł peptide bond contribution to ε near 288 nm; and (3) anti-octant perturbation of oxirane ring.
4 |
cyclo (Aib-L-Phe-D-Pro-2S,9S-Aoe) |
0.26 288 |
|
5 |
cyclo (Aib-L-Phe-D-Pro-2S,9R-Aoe) |
C 0.25 |
288 |
6 |
cyclo (D-Pro-L-Ala-D-Ala-L-Aoe) |
0.24 |
288 |
7 |
cyclo (D-Phe-L-Leu-L-Pip-L-Aoe) |
0.23 |
288 83 |
|
|
|
|
|
|
NH2 |
O |
where Aib |
D |
˛-aminoisobutyric acid, Pip |
D |
pipecolic acid and Aoe |
D |
HO |
O |
2 (CH2 )5 |
9 |
||||||
|
|
|
O |
H |
The identity of the Cotton effects of natural peptides 6 and 7 with the synthetic peptide 4 having a known oxirane configuration allowed for assignment (9S) of the absolute
configuration of the L-Aoe residue83.
O |
|
|
O |
|||||||
|
|
|
|
|
|
|||||
CH3 |
|
CCH2 CH2 SCH2 |
CH |
|
CHCH2 SCH2 CH2 |
|
CCH3 |
|||
|
|
|||||||||
|
|
|
|
|||||||
|
|
|
|
OR |
|
OR |
|
|
|
|
R = H +0.026 (285), |
|
|
−0.051 (245)8 4 |
|||||||
R = CH3 (CH2 )2 CH |
|
|
+0.004 (290)8 5 |
|
||||||
O
n
O
−1.56 (275)8 6
O |
|
|
|
CH2 CH2 CCH2 CH2 SCH2 CH |
CHCH2 S |
O |
n |
O |
|
−0.014 (285)8 5 |
CH(CH2 )2 CH3 |
|
|
O |
|
+0.12 (284)
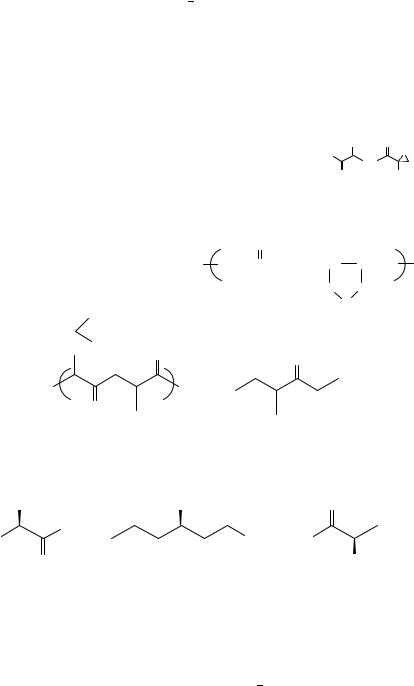
The CD data for a series ˛- or ˇ-deuterated aliphatic aldehydes (8, 9) and ketones (10) were reported87.
|
D |
|
|
|
D |
O |
|
O |
|
|||
|
R |
H |
|
|
|
|
|
|
|
H |
R |
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|||
|
|
O |
|
|
|
|
|
|
|
|
|
D |
(8a) |
R = n-Pr |
−0.034 (298) |
(9) |
+0.006 (298) |
|
|
(10a) |
R = n-Pr |
−0.079 (298) |
|||
(8b) |
R = i-Pr |
−0.028 (298) |
|
|
|
|
|
|
|
(10b) |
R = i-Pr |
−0.098 (298) |
The n ! Ł Cotton |
effects for |
8 |
|
10 |
were |
consistent with the |
preferred (ca |
|||||
|
||||||||||||
1 kcal mol 1) eclipsed conformation of the carbonyl/˛-alkyl moiety as shown in the octant projections below.
The ‘anti-octant’ or dissignate behavior of deuterium was discussed earlier (Section II.F), and it operates as expected in compounds 8 10. The octant representations
178 |
|
|
|
|
|
|
|
Stefan E. Boiadjiev and David A. Lightner |
|
|
|
|
|
|
||||||||
+ |
D |
− |
+ |
H |
H |
|
|
− |
+ |
|
|
H |
− |
|||||||||
|
|
|
|
|
|
|
|
|
|
|||||||||||||
R |
|
|
|
O |
|
H |
|
Pr |
|
|
|
|
O |
|
H |
R |
|
O |
|
|
|
Me |
|
|
|
|
|
D |
H |
|
|
D |
|||||||||||||
− |
H |
+ |
− |
+ |
− |
|
|
+ |
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
(8) |
|
|
|
|
|
(9) |
|
|
|
|
(10) |
|
|||||||||
show also that when the carbonyl perturber is displaced one carbon farther away, as in the aldehyde 9 compared to 8, the ε magnitude is decreased five-fold, thus demonstrating the ‘proximity rule’ in these cases. Changes of solvent polarity did not appear to affect the conformational preference in 8 10, though variable-temperature effects were observed.
B. Cyclic Ketones and Aldehydes
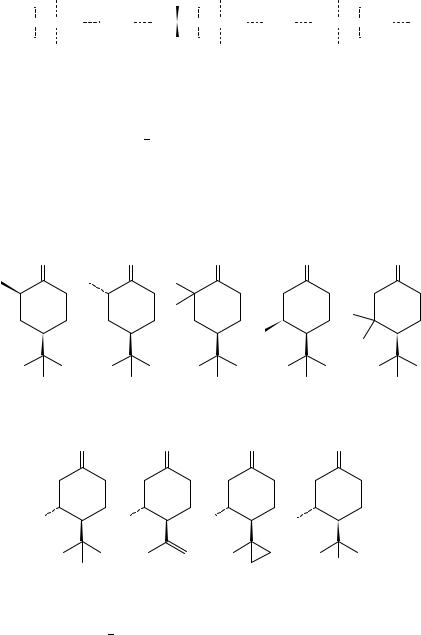
Djerassi and coworkers88 showed the extraordinary sensitivity and utility of the chiroptical methods in detecting subtle conformational changes by CD measurements on chiral 4-tert-butylcyclohexanones.
O O O O O
(11) |
(12) |
(13) |
(14) |
(15) |
−0.46(298)8 8 |
+1.33(296)8 8 |
+1.36(295)8 8 |
−0.17(297)8 8 |
−0.39(303)8 8 |
O |
O |
O |
O |
|
|
|
|
Br |
(16) |
(17) |
(18) |
(19) |
+3.39(299)8 8 |
−0.45(297)8 8 |
−0.70(295)8 8 |
+2.71(297)8 8 |
While the ketones 11 15 are anchored by an equatorial tert-Bu group in the preferred chair conformation, and the octant contributions of ˛- or ˇ-methyl substituents follow according to expectation, the CD of ketone 16 was unanticipated. The predicted CD of 16 in a chair conformation (with ˇ-equatorial methyl group) was weak and negative as was
5. Chiroptical properties of compounds containing CDO groups |
|
179 |
|||||
ε ca |
. |
ε |
|
|
19 were calculated |
approxi- |
|
found for 17 and 18 ( |
0 5). The |
|
of compounds 11 |
|
88 |
. The |
|
mately from the reported rotational strength using the relationship: [R] D 3.32 Ð ε |
|||||||
observed strong positive Cotton effect for 16 and for the similar bulky substituted 19 led to a reasonable assumption that the twist-boat form is of lower energy, confirmed by an empirical force-field calculation. The unusually large positive n ! Ł CD of 16 indicates that the presence of equatorial substituent adjacent to the 4-tert-butyl blocking group causes the twist-boat conformation to become energetically preferred due to release of an unfavorable nonbonded steric repulsion between the 4-equatorial tert-Bu and 3-equatorial Me interaction.
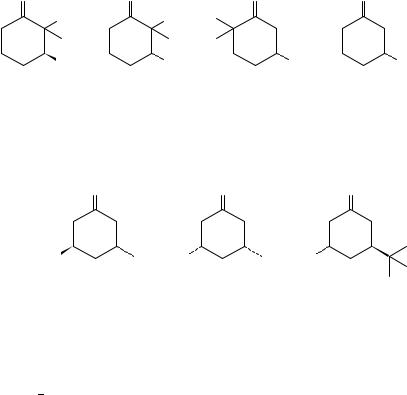
1H-NMR and CD spectroscopy have been used to determine the equatorial axial equilibrium in ˇ-heteroatom-substituted cyclohexanones89.
O |
|
|
|
O |
|
|
|
O |
O |
|
|
|
R |
|
|
R |
|
|
|
|
|
|
|
|
X |
|
|
X |
|
|
X |
|
SR |
||
|
R = CH3 |
or CD3 |
|
|
|
|
|
|
|
||
X |
∆ε |
|
X |
∆ε |
|
X |
∆ε |
R |
∆ε |
||
OH |
+0.07 |
|
|
F |
+0.33 |
|
OAc |
+0.06 |
|
Et |
+0.41 |
OAc |
−0.67 |
|
Cl |
+1.69 |
|
Me |
+1.388 9 |
|
4-t-BuC6 H4 |
+2.859 0 |
|
OMe |
−0.808 9 |
|
SEt |
+2.97 |
|
|
|
|
|
|
|
|
|
|
|
Me |
−0.518 9 |
|
|
|
|
|
|
O |
O |
O |
EtS |
EtS |
EtS |
+1.418 9 |
−2.858 9 |
−1.278 9 |
[All data are for n → π (296−301 nm) Cotton effect measured in EPA at RT.]
Comparison with monosubstituted cyclohexanones showed that in the case of F, OH, OMe, OAc and Me substitution, the 3-keto group enhanced the axial preference of the substituent, the effect being greater for more electronegative substituents. Less electronegative substituents (Cl, Br and SR) showed a decreased axial preference89.
The octant rule holds for 2-methylpiperidin-4-one (20) and trans-decahydroquinolin-4- ones (21 23).
The CD of bicyclic ketone 2495 as hydrochloride as well as 2596 was compared to that of their carbocyclic analog (C)-norcamphor (26)49, and the absolute configuration was established as (1R,4S) and (1R,4R) for 24 and 25, respectively. Comparison between ( )- 24ÐHCl and (C)-26 was based on an earlier finding that the relative geometry of the lone pair on the N atom and the C˛ CO bond greatly influences the CD of ˛-aminoketones, whereas the coupling between n ! Ł and ! Ł transitions is removed by protonation in ( )-24ÐHCl.
The CD of all trans-fused ketones belonging to the perhydro-naphthalene, -anthracene, -naphthacene and -phenanthrene has been reported. These studies provided the pure ring
180 |
|
|
|
Stefan E. Boiadjiev and David A. Lightner |
|
|
|
||||||||
O |
|
R2 |
|
|
O |
|
SEt O |
|
|
|
S |
O |
|||
|
|
|
R1 |
|
|
|
|
|
R |
|
|
R |
|
|
R |
|
|
|
R1 = H91 |
|
|
|
|
|
|
|
|
|
|
|
|
R2 |
= H |
+0.94 |
(290) |
|
|
R = H |
|
−1.82 (288), |
R = H |
+3.26 (289), |
|||||
R2 |
= SEt |
+1.39 |
(291), |
+0.85 (248) |
|
|
+0.39 (249) |
|
R = OMe |
+2.55 (247) |
|||||
R2 |
= SBu-t |
+1.44 |
(290), |
+1.03 (248) |
R = OMe |
|
−1.58 (289), |
+3.19 (288), |
|||||||
2 |
= SPh |
+1.15 |
(295), |
+1.36 (260) |
|
|
+0.45 (247) |
9 1 |
|
|
+2.97 (243)91 |
||||
R |
|
|
|
|
|
|
|
|
|||||||
|
|
|
R1 = OMe91 |
|
|
|
|
|
|
|
|
|
|
||
|
= H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R2 |
+0.83 |
(292) |
|
|
|
|
|
|
|
|
|
|
|
||
R2 |
= SEt |
+1.34 |
(290), |
+0.83 (248) |
|
|
|
|
|
|
|
|
|
||
R2 |
= SBu-t |
+1.40 |
(291), |
+1.11 (249) |
|
|
|
|
|
|
|
|
|
||
R2 |
= SPh |
+1.12 |
(294) |
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
O |
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
HC |
|
|
|
|||||
|
|
CHSCH2 CH |
CHCH2 SCH |
|
|
|
CHSCH2 CH CHCH2 S |
||||||||
|
|
Ph |
|
OR |
OR |
Ph |
|
|
Ph |
|
|
Ph |
OR |
OR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
n |
|
|
+0.37 (293), |
−1.25 (244) |
|
R = H |
|
|
+0.30 (294), |
−0.30 (247)84 |
||||||
|
|
+0.08 (290) |
|
|
|
R = CH3 (CH2 )2 CH |
+0.15 (300)8 5 |
|
|||||||
|
|
|
O |
|
|
|
O |
|
|
|
|
O |
|
|
|
|
|
|
|
|
R |
|
|
|
CN |
|
|
|
|
|
R |
|
|
|
N |
|
|
|
N |
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
|
CH3 |
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
||
|
R = CN |
|
+1.17 (300) |
|
+1.04 (300)92 |
|
R = CN |
−0.88 (299) |
|||||||
|
R = COOMe +1.44 (298)92 |
|
|
|
|
|
R = COOMe −0.91 (300)92 |
||||||||
|
|
|
O |
|
|
|
O |
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
N |
|
|
|
N |
|
|
|
|
H |
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
R |
|
||
|
|
|
|
|
|
|
|
|
(21) R = H |
+0.76 (296) |
|||||
|
|
|
|
|
|
|
(20) |
|
(22) R = (S)-CH3 CH2 (CH3 )CH |
||||||
|
|
|
|
|
|
|
|
|
|
|
+0.73 (293) |
||||
|
|
|
|
|
|
+0.16 (296)9 3 |
|
|
|
||||||
|
−0.15 (300)9 2 |
|
(23) R = (S)-PhCH2 (CH3 )CH |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
+0.68 (304)9 4 |
|||
5. Chiroptical properties of compounds containing CDO groups |
181 |
|||||
O |
|
O |
|
|
O |
|
|
|
|
|
O |
|
|
N |
N |
|
|
|
|
|
|
HCl |
O |
|
|
|
|
(−)-(24) |
(−)-(24.HCl) |
(−)-(25) |
(+)-(26) |
|
||
−2.1 (309) |
−0.012 |
(313), |
−0.78 (298) |
−0.25 |
(305), |
|
|
+0.123 |
(282) |
|
+0.15 |
(280) |
|
additive contributions (including from the front octants) of third and fourth annelated unsubstituted rings to the previously proposed empirical rules43,49,97,98 for extended decalones.
|
H |
H |
H |
||||
|
R |
R |
S |
||||
|
S |
R |
R |
||||
|
|
|
|
O |
H |
|
|
|
H |
|
|
H |
|
|
|
|
|
O |
|
O |
|||
|
|
|
|
|
(27) |
|
|
Hexane |
+0.81 (296), +3.6 (188)4 3 |
−1.12 (297), −0.5 (190)4 3 |
−1.43 (297), −2.72 (192)9 9 |
||||
MeOH |
+0.95 (292)4 3 |
−1.37 (290)4 3 |
−1.85 (293)99 |
||||
CF3 CH2 OH |
−1.9 (189)100 |
+1.0 (190)100 |
|
|
|
||
H |
|
|
H |
|
|
H |
|
H |
|||||
R |
|
|
S |
|
|
R |
|
S |
|||||
R |
|
|
S |
|
|
|
R |
|
|
R |
|||
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
H |
|
|
H |
|
|
|
|
H |
|
H |
|||
|
|
|
|
O |
|
|
|
|
|
|
|
||
Hexane |
+0.76 (295), |
+3.9 (187)101,102 |
−1.37 (297), |
+1.5 (185)4 3 |
|||||||||
MeOH |
+1.01 (290), |
<0 (<196)101,102 |
−1.86 (290)4 3 |
||||||||||
CF3 CH2 OH −1.9 (188)100 |
+2.8 (194)100 |
|
|
||||||||||
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
S |
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
R |
H |
|
|
|
||
R |
|
|
|
|
|
||||||||
|
|
|
|
|
|
||||||||
|
|
|
S |
|
|
|
|||||||
H |
|
|
|
|
|
H |
S |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
S |
||||||
|
|
|
|
|
|
|
|
||||||
|
O |
|
|
|
|||||||||
|
|
|
|
|
|
|
|
||||||
(28) |
|
|
|
|
|
|
|
H |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
||||
−0.53 (299), −3.02 (189)9 9 |
|
|
|
|
O |
||||||||
Hexane |
+1.20 (296), |
+1.40 (189) |
|||||||||||
−0.54 (295)9 9 |
|
|
|
|
|
||||||||
|
|
|
|
|
MeOH |
+1.67 (291), −2.10 (192)10 2 |
|||||||
182 |
|
|
Stefan E. Boiadjiev and David A. Lightner |
|
|
|
|
|
||||||
|
|
|
HH |
|
|
|
H |
|
|
|
|
|
||
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
H |
S |
R |
|
|
|
S |
S |
|
|
|
|
|
||
|
|
R |
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
H |
O |
|
|
H |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
O |
||||
|
−0.56 (297), |
+0.2 (193) |
|
+1.26 (300), |
+3.55 (190) |
|||||||||
|
−0.72 (288)102 |
|
+1.37 (296)99 |
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
O |
|||||
|
|
H |
|
|
|
|
|
|
|
|
|
|
||
|
|
H |
S |
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
S |
|
|
|
|
|
|
|
R |
R |
|
|
|
|
|
|
|
|
|
||
|
|
H |
|
|
S |
S |
|
|
|
|
|
|||
|
|
S |
|
|
|
|
|
|
H |
|||||
|
|
|
|
|
|
|
|
|
||||||
|
|
H |
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
||
Hexane −0.47 (296), −0.7 (190) |
|
+1.40 (298), −1.0 (195) |
||||||||||||
MeOH −0.75 (293)103 |
|
|
+1.70 (290)103 |
|
|
|
|
|
|
|||||
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
H |
|
|
|
H |
|
S |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
S |
R |
|||||||
|
|
R |
O |
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
R |
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
R = CO2 H |
|
−1.09 (289) |
|
|
|
|
H |
|
|
O |
||||
|
|
|
|
|
|
|
|
|||||||
R = CH2 OH |
|
−1.50 (293) |
|
|
|
|
|
|
|
|
|
|
||
R = CO2 Me |
|
−1.23 (292), −1.89 (208) |
|
Hexane |
+1.46 (295), <0 (195) |
|||||||||
|
|
−1.38 (290), |
−0.38 (208)99 |
|
MeOH |
+2.14 (293), |
|
|
−2.2 (196)104 |
|||||
|
|
|
|
|
H |
H |
H |
|
|
|
|
|
|
|
|
|
|
|
|
R |
R |
S |
|
|
|
|
|
|
|
|
|
|
|
|
R |
R |
R |
|
O |
|
|
|
|
|
|
|
|
|
|
H |
H |
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
−1.55 (298), |
+2.0 (187) |
|
|
|
|
|
|
||
|
|
|
|
|
−2.05 (291)102 ,105 |
|
|
|
|
|
|
|
||
The contribution of the methyl substituent in 8-methyl-1-decalones 27 and 28 is octant consignate but different in magnitude for the axial and equatorial methyl group, though they are nearly symmetrically located with respect to the horizontal carbonyl plane99.
5. Chiroptical properties of compounds containing CDO groups |
183 |
|||
|
|
OMe |
|
|
|
OAc |
|
OMe |
|
|
CO2 Me |
|
|
|
|
|
|
|
|
H |
OAc |
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
OAc |
|
|
|
O |
|
N |
O |
|
O |
|
|
||
|
|
|
||
|
|
|
|
|
|
|
Me |
|
|
+0.14 (298)106 |
−0.19 (297)107 |
−0.42 (303), |
+0.32 (269) |
|
|
Methiodide |
−0.31 (330), |
+0.39 (273)108 |
|
X-ray analysis of hydrindanone 29 showed a cis ring fusion. The absolute configuration of 29 was proposed by applying the octant rule109. The absolute configuration of the trans- fused hydrindanone 30 was correlated by chemical transformations of ( )-carvone. The ketone 30 CD is in accord with the octant rule110.
H
|
|
H |
|
H |
|
O |
O |
|
|
|
|
(29) |
(30) |
|
|
|
+0.37 (304) |
+1.33 (294) |
|
|
|
|
O |
H |
|
|
|
H |
|
HO |
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
H |
H |
|
|
|
O |
|
OH |
O |
H |
|
|
|
|
|||
|
|
+0.94 (318)111 |
−1.82 (293)112 |
|
H |
|
|
O |
CH2 OAr |
|
|
|
||
|
|
|
O |
|
H |
|
|
OH |
|
O |
|
|
O |
|
+1.07 (286)112 |
|
|
+0.19 (318)113 |
−0.10 (338), +0.87 (298), |
|
|
|
sh |
−1.55 (230)114 |
184 |
Stefan E. Boiadjiev and David A. Lightner |
|
||
|
CH2 OAr |
|
CH2 OAr |
|
|
|
|
MeO |
|
|
|
|
Ar = |
|
O |
|
O |
|
O O |
|
|
|
|
OMe |
+0.80 (340), |
+2.40 (295)114 |
−0.44 (345), |
+1.12 (299), |
|
|
|
−4.40 (210)114 |
|
|
|
COOH |
|
eq |
ax |
|
|
CH2 OAr |
||
|
|
CH2 OAr |
||
|
|
|
|
|
|
|
|
OHCCH2 CH2 |
|
O |
|
O |
|
|
+0.04 (318), |
−0.23 (289)114 |
+0.05 (335), |
−2.53 (290)114 −0.24 (350), |
−0.85 (293)114 |
|
CH2 OAr |
|
CH2 OAr |
CH2 OAr |
O |
O |
|
O |
|
|
−0.42 (330), |
+0.05 (295), |
−0.58 (328), |
+0.04 (295), |
−0.50 (330), −4.5 (204)115 |
|
−0.50 (245), −19.5 (203)115 |
−10.3 (203)115 |
|
|
||
|
|
|
Ar |
= |
|
|
|
|
|
O |
O |
|
eq |
|
|
|
|
|
CH2 OAr |
|
CH2 OAr |
H |
|
|
|
|
|
|
|
O |
|
O |
|
|
O |
|
OH |
|
O |
H |
|
|
|
|
|
||
−0.42 (330), +1.60 (294)115 |
−1.20 (326), |
+3.10 (290)115 |
+1.07 (295)116 |
|
|
An interest in anomalous CD properties of 4,4-dimethyl-3-keto steroids and 4,4,8ˇ- trimethyl-3-keto steroids117,118 continued in the studies of Tsuda and coworkers on onoceranediones119,120. Analysis of the CD spectra of 31 35 in methanol and dioxane led to the conclusion that the A-ring conformation in solution is in equilibrium between chair
