
|
|
|
|
9. Liquid crystals with XDY groups |
|
453 |
|||||||||
|
|
|
|
|
CH3 |
|
CH3 |
|
|
||||||
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
(CH2 )7 |
|
(CH2 )n |
|
|
||||||||||
|
|
(CH2 )9 |
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
NH |
(H17C8 )S |
S(C8 H17) |
|||||||||||
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
O |
|
|
|
NCH3 |
|||
|
NH |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|||||||||
|
|
|
O |
HO |
|
|
|
OH |
HO |
|
|
|
OH |
HO |
OH |
|
|
|
|
|
|
|
|
||||||||
|
|
|
|||||||||||||
|
|
|
OH |
|
|
|
OH |
|
|
|
OH |
OH |
|||
|
|
|
|
|
|
|
|
||||||||
|
|
|
OH |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
OH |
|
|
|
|
OH |
|
|
|
|
OH |
|
OH |
|
|
|
|
|
|
|
|
|
|
||||||
|
|
CH2 OH |
|
|
|
CH2 OH |
|
|
|
CH2 OH |
|
CH2 OH |
|||
|
|
|
|
|
|||||||||||
|
|
(a) |
|
(b) |
|
(c) |
|
(d) |
|||||||
C 99 L 151 I |
Cl 79 C2 96 (C3 150) S 156 I |
C 90 (SA 61) I |
C 102 SX 118 I |
||||||||||||
|
|
|
|
|
|
|
|
|
|
(n = 7) |
|
|
|||
FIGURE 31. (a) N-Decylribonamide, (b) n-octylgluconamide, (c) 1-deoxy-1-(N-methyloctylamido)-D- glucitol and (d) D-glucose-S,S-dioctyl acetal
OR |
|
OR |
|
O |
|
O |
|
RO |
O |
|
|
RO |
RO |
OR |
|
|
|
||
OR |
|
OR |
|
R = COC9 H19 |
|
||
|
C66 Dho 90 I |
|
|
|
(a) |
|
|
|
|
OR |
|
OR |
O |
||
RO |
O |
||
OC12 H2 5 |
|||
|
|
||
RO |
O |
HO |
|
OR |
|||
|
|||
|
OR |
||
|
|
||
|
|
R = C11H2 3 |
|
|
|
C74 D 93 I (°C) |
|
|
|
(b) |
FIGURE 32. Examples of first discotic LC-CH’s
large number of important data related with alicyclic LC-CH’s. Following the accessible data, different kinds of glycosides were described in literature:
ž(un)-protected cyclic sugar portions with aliphatic glycosides,
ž(un)-protected cyclic sugar portions with alicyclic carbohydrate glycosides,
ž(un)-protected cyclic dior tri-saccharides,
ž(un)-protected alicyclic dior trisaccharides.

454 |
T. Hanemann and W. Haase |
Simple glycosides represented in the ˛/ˇ-D-glucopyranose-derivatives are shown in Figure 33a; the related mesomorphic data are given in Table 14. Solid state polymorphism can only be observed in case of the ˇ-glycosides, and all compounds have only a SA phase. The typical odd even effect is present, and the ˛-glycosides possess higher transition temperatures and a larger phase range than their ˇ-anomers. The related mannopyranosides (Figure 33b) and galactopyranosides (Figure 33c) show only the SA phase, the clearing temperatures of the mannosides in comparison to the glucosides are higher, the melting temperatures are lower (Table 15). The ˇ-galactosides possess higher transition temperatures than the ˇ-glucosides. Again, the latter only show solid state polymorphism. So one can conclude that axially oriented hydroxy moieties stabilize the SA phase in an unequivocal manner due to a stronger intermolecular hydrogen bridge to the next smectic layer. An increasing number of sugar units like dior trisaccharides leads in general to an increase in the phase-transition temperatures; in all cases the SA phase occurs106. A different behaviour is expected considering the alicyclic glycosides. Table 16 lists a series of homologues of the mentioned 1-deoxy-1-(N-methylalkylamido)-D-glucitol (Figure 31c). An odd even effect cannot be observed, and without exception the phase transition temperatures rise with increasing chain length. The amphilic character of these compounds is
OH |
|
OH |
O |
|
O |
HO |
HO |
OR |
HO |
HO |
|
OH |
|
OH |
OR |
|
|
|
(a) |
|
OH |
HO |
OH |
|
|
|
OH O |
|
O |
HO |
HO |
|
HO |
|
|
|
|
|
|
|
OH |
OR |
|
OR |
(b) |
|
(c) |
FIGURE 33. (a) ˛/ˇ-D-Glucopyranoside, (b) ˛-D-mannopyranoside and (c) ˇ-D-galactopyranoside
TABLE 14. Mesomorphic data of ˛/ˇ-D-glucopyranosides with different chain lengths (°C)
n-Heptyl |
C |
53 |
SA |
99 |
I |
C1 |
56 |
C2 |
59 |
SA |
69 |
I |
n-Octyl |
C |
72 |
SA |
118 |
I |
C |
67 |
SA |
106 |
I |
|
|
n-Nonyl |
C |
65 |
SA |
130 |
I |
C1 |
51 |
C2 |
68 |
SA |
113 |
I |
n-Decyl |
C |
76 |
SA |
138 |
I |
C1 |
65 |
C2 |
70 |
SA |
136 |
I |
n-Dodecyl |
C |
77 |
SA |
151 |
I |
C1 |
55 |
C2 |
63 |
C1 |
80 |
SA 143 I |
n-Hexadecyl |
C |
108 |
SA |
175 |
I |
C1 |
78 |
C2 |
110 |
SA |
145 |
I |
TABLE 15. Mesomorphic data of ˛-D-mannopyranosides and ˇ-D-galactopyranosides (°C)
n-Octyl |
C |
55 |
SA |
134 |
I |
C |
98 |
SA |
133 |
I |
|
|
n-Decyl |
C |
65 |
SA |
153 |
I |
C1 |
82 |
C2 |
94 |
SA |
157 |
I |
n-Dodecyl |
C |
74 |
SA |
162 |
I |
C1 |
55 |
C2 |
99 |
SA |
166 |
I |
9. Liquid crystals with XDY groups |
455 |
||||
TABLE 16. |
Phase range of various 1-deoxy-1-(N-methyl- |
|
|||
alkylamido)-D-glucitols (MEGA n-2) |
|
|
|
|
|
|
|
|
|
|
|
n |
Name |
Phase range |
|
||
|
|
|
|
|
|
4 |
MEGA6 |
C 71 I |
|
||
5 |
MEGA7 |
C 87 I |
|
||
6 |
MEGA8 |
C 78 I |
|
||
7 |
MEGA9 |
C 90 |
(SA 61) I |
|
|
8 |
MEGA10 |
C 91 |
(SA 88) I |
|
|
9 |
MEGA11 |
C 92 |
SA 113 I |
|
|
10 |
MEGA10 |
C 94 |
SA 125 I |
|
|
shown by forming lyotropic phases with water (n D 8 11, 17) due to the large number of polar hydroxy groups. The introduction of an ethylendiamine bridge between the glucose moiety and the alkylamido portion destroys the mesomorphism, so that only solid state polymorphism occurs, while methylation of one amino nitrogen restores the liquid crystalline behaviour101. Peralkylation of the unprotected hydroxy groups of the pyranoses allows for the synthesis of discotic carbohydrates. Per-n-decanoyl-˛/ˇ-D-glucopyranose possesses only a monotropic discotic phase within a very small range:
˛ : C 34 D 27 II ˇ : C 38 D 32 I [°C]
X-ray investigations of the crystal phase of a series of glycosides lead to two different models describing the molecular arrangement in the important SA phase107 109. Comparing the measured value of the layer thickness using X-ray analysis, it is obvious that two molecules arrange in a bilayered way. One model (Figure 34, left side) describes the dimer with a partially overlapping core consisting of the cyclic carbohydrate moiety with the interacting hydroxy groups via hydrogen-bonding. The elongated aliphatic chains form the outside with weak van der Waals interaction between the smectic layers. The second approach suggests the opposite, i.e. the aliphatic chains set up the core and the hydrophilic carbohydrate portions form the outside boundary to the next layer, as in cell membranes (Figure 34, right side). The latter model is in agreement with investigations on the given fluid lamellar lyotropic phase. In general, the hydrogen bridges are responsible for the bilayer formation (the SAd phase); polar solvents like water
Carbohydrate |
|
Carbohydrate |
Carbohydrate |
|
|
|
Carbohydrate |
Carbohydrate |
Carbohydrate |
|
|
|
Carbohydrate |
Carbohydrate |
Carbohydrate |
|
|
|
Carbohydrate |
Carbohydrate |
|
Carbohydrate |
|
FIGURE 34. Proposed models of bilayer formation in the SAd phase
456 T. Hanemann and W. Haase
alcohols interpenetrate the direct carbohydrate/carbohydrate interaction and increase the distance between neighbouring molecules in one layer108. Even more complex systems like n-octyl-˛-glucosylarabonamid or related molecules110,111 can be described, and the obtained structural data are in good agreement with X-ray investigations (Figure 35)112.
˚ |
˚ |
The total molecular extension is 22.5 A, the length of the aglycon is 17.9 A and that |
|
˚ |
˚ |
of the octyl-group is 10.9 A. The measured |
d-value for the layer thickness is 33.7 A, |
and Figure 36 gives an impression of the assumed molecular arrangement in the SAd phase. The most important advantage of carbohydrates is their unlimited presence as natural raw material. A large number of natural products contain sugar moieties, therefore it is a challenge to modify or combine these molecules with other building blocks thus introducing new interesting properties. An actual result is the synthesis of liquid crystalline umbelliferyl-ˇ-D-glucosides113 (Figure 37, top). As in other lc glucosides as smectic A phase occurs, but a common bilayer formation has not been detected. With increasing chain length the mesomorphic behaviour changes from non-liquid crystalline (R D C7H15) over monotropic to an enantiotropic SA phase.
The last examples contain the azomethine or Schiff-base unit114. Simple pyran derivatives cannot be treated as carbohydrates because the typical amphiphilic character due to the large number of hydroxy moieties is missing. On the other hand, pyran is the central core of all hexose-carbohydrates. The listed compounds contain the classical structural subunits as mentioned in the beginning, so they represent a link between the standard lc’s and the carbohydrate family (Figure 37, bottom) . The authors discuss four different types via variation of the bridging units X,Y and the terminal aliphatic chains (Table 17). The combination of the phenol ether with the CHDN group allows only for monotropic behaviour; the turnover of the azomethine results in stable enantiotropic nematic phases. All the amino-bridged derivatives possess an SA phase, and in the case of short aliphatic chains the nematic phase is present too. The given pyran derivatives demonstrate in a typical manner how the change of substituents influences the liquid crystalline properties. In the following we summarize the basic structure elements of liquid crystalline carbohydrates and try to assume some structure-mesomorphic property relationships on the basis
|
|
|
OH |
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
HO |
|
|
OH |
O |
||||
|
|
HO |
|
|
||||||
|
|
OH |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
|
|
|
O |
|
|
|
NHC8 H17 |
|||
|
|
|
OH |
|
OH |
|||||
FIGURE 35. Structure of n-octyl-˛-glucosylarabonamid |
|
|
|
|
|
|
||||
|
TABLE 17. Schematic list of mesomorphic properties |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
X |
Y |
Z |
|
|
|
Phase behaviour |
|||
|
|
|
|
|
|
|
|
|
|
|
|
O |
CHDN |
OCnH2nC1, n D 6 |
|
10 |
|
|
C, (N), I |
||
|
|
|
|
|||||||
|
O |
NDCH |
OCnH2nC1, n D 4 |
|
12 |
|
|
n D 4: C, SA, N, I |
||
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
n > 4: C, N, I |
||
|
NH |
NDCH |
OCnH2nC1, n D 1 |
|
9 |
|
|
n D 1,2: C, SA, N, I |
||
|
|
|
|
|||||||
|
NH |
CHDN |
OCnH2nC1, n D 4 |
|
|
|
|
n > 3: C, SA, I |
||
|
|
10 |
|
|
n D 4 |
|
6: C, SA, N, I |
|||
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
n > 6: C, SA, I |
||

FIGURE 36. Assumed arrangement in the SAd phase of n-octyl-˛-D-glucosylarabonamid
457
458 |
|
T. Hanemann and W. Haase |
|
OH |
|
|
O |
|
HO |
|
O |
HO |
|
|
|
|
|
|
OH |
|
|
|
R |
R = C9 H19 |
C160 (SA 159) I (°C) |
|
R = C13 H2 7 |
C163 SA 200 I |
O |
O
X |
Y |
Z |
O |
|
|
X = NH, O |
|
|
Y = N |
CH, CH |
N |
Z = H, Cl, Br, NO2
R = O(CH2 )nCH3 (n = 0 − 11)
FIGURE 37. Top: First liquid crystalline umbelliferyl glycosides and bottom: simple pyran derivatives
of the investigated systems:
ždifferent carbohydrates (cyclic, alicyclic, mono, di, oligosaccharides) possible,
žlong aliphatic chains (n > 6) attached as glycosides (O, C, S, N type) favourable,
ž˛-/ˇ-anomers possible,
žhydroxy groups protected or unprotected,
žacetalization of the carbonyl group destroys lc properties; exception: dithioacetals,
žterminal -OH, -Cl, -CN(!) moieties destroy lc properties.
For the next years a rapid increase in this field of research is expected.
D. Ferroelectric and Antiferroelectric Liquid Crystals
In solid state all the 10 pyroelectric crystal groups allow in principle for bistable switching behaviour. This is the proper ferroelectricity. Under certain conditions ferroelectricity (improper) can be realized in liquid crystals. This was shown by Meyer and coworkers115 in 1975. Since that time intense activities have been initiated, applying this property for flat-panel devices, switches, light modulators etc. In principle, three effects can be observed and used:
žSurface Stabilized Ferroelectric Liquid Crystals (SSFLC)116: Here all three vectors
of spontaneous polarization (PS) are initially aligned by surface effects in thin cells (ca 2 m). The switchability is due to 180° rotation of the PS vectors on a cone.
žDeformed Helical Ferroelectrics (DHF)117: In a thicker cell (ca 5 m) the surface stabilization no longer dominates and a helical structure is formed with the helix axis parallel to the glass plate. The external electrical d.c. field can influence and modulate the helix.
9. Liquid crystals with XDY groups |
459 |
žElectroclinic effect118,119: Close to the phase transition from the tilted SmCŁ phase to the orthogonal SmAŁ phase the tilt angle becomes soft (soft mode). Consequently, one can realize faster switching times than in SSFLC and DHF cells.
Antiferroelectric LC’s with tristable switching have been known for the last ten years120,121. The chemistry of ferroelectric and antiferroelectric liquid crystals follows the rules valid for calamitic LC’s, but with some additional constraints:
žThe structure must build up a tilted phase, commonly a smectic C phase. The molecules
contain at least one chiral centre and the symmetry cannot be assumed as C2h symmetry, but only as C2v symmetry. This is consistent with a polar twofold axis perpendicular to the long molecular axis.
žThe molecule dipole moment is oriented perpendicular to the long axis.
The first investigated ferroelectric LC is DOBAMBC (p-decyloxybenzylidene-p0- amino-2-methylbutylcinnamate)122, the structure is presented in Figure 38. DOBAMBC with a small spontaneous polarization at Tc T D 10 K of ca 3 nC cm 2 shows a typical behaviour for compounds with X D Y ( CDN , CDC , CDO ) groups.
Azomethine derived ferroelectric liquid crystals: As DOBAMBC, many ferroelectric LC’s were prepared utilizing amyl alcohol as the chiral source. The reason for the small spontaneous polarization of DOBAMBC is the separation between the CDO dipole moment and the chiral carbon. This effect can be explained by the intramolecular rotation or vibration of the carbonyl dipole moment relative to the chiral carbon, because they are not adjacent. There are some rules linking the molecular structure and the direction of the spontaneous polarization (minus or plus). In order to reduce the separation between the carbonyl dipole moment and the chiral carbon, ferroelectric LC’s were synthesized utilizing a secondary alcohol as the chiral source. Ferroelectric LC’s with large spontaneous polarizations have large dipole moments at the chiral centre. Ferroelectric LC’s with halogen or nitrile units connected directly to the chiral carbon were synthesized from chiral lactic acids or amino acids.
Azo and azoxy series of ferroelectric liquid crystals: Several of these were prepared; one of them is shown in Figure 39. Unfortunately, these materials are not so stable and therefore useless for practical applications.
|
|
|
|
|
|
|
|
CH3 |
H21C10 O |
CH N |
|
CH |
CH COO |
CH2 CH C2 H5 |
|||
|
|
|
|
|
|
|
|
* |
FIGURE 38. Chemical structure of DOBAMBC |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
CH3 |
RO |
N N |
|
|
|
O CH2 |
CH C2 H5 |
||
|
|
|
|
|
|
|
|
* |
|
or with |
|
N |
|
N |
|
group |
|
|
|
|
|
|
||||
|
|
|
|
|||||
|
|
|
|
|
||||
O
FIGURE 39. Azo and azoxy compounds (R D alkyl)
460 |
T. Hanemann and W. Haase |
|
|
|
|
CH3 |
|
RO |
COO CH2 |
CH |
C2 H5 |
|
|
* |
|
FIGURE 40. Chemical structure of 4-alkoxybiphenyl-40 -carboxylate compounds |
|
||
|
O |
O |
|
H17C8 O |
|
|
|
|
|
|
|
|
O |
|
C6 H13 |
O *
H
CH3
FIGURE 41. Chemical structure of MHPOBC
|
|
O |
|
|
R1 |
C |
C |
O |
|
|
|
O |
|
|
|
|
|
O |
R * |
|
|
|
|
2 |
FIGURE 42. Chemical |
structure |
of an antiferroelectric |
liquid crystal (R1:alkoxy, R2 D |
|
4-methylheptyloxycarboxyl) |
|
|
|
|
FIGURE 43. Structures of the ferroelectric and antiferroelectric smectic CŁ phases in their unwound states

9. Liquid crystals with XDY groups |
461 |
Biphenyl and aromatic ester series of ferroelectric liquid crystals: Numerous 4-alkoxy (or 4-alkyl) biphenyl-40-carboxylate compounds were synthesized. The basic chemical structure of these is presented in Figure 40. These compounds show ferroelectric phases at room temperature, but their spontaneous polarizations are relatively small.
Several polycyclic ferroelectric LC’s were prepared by utilizing amyl and secondary alcohols as the chiral source in which biphenyl and phenyl rings were connected via an ester linkage. Also, various heterocyclic ferroelectric LC’s were synthesized by the introduction of chiral units into heterocyclic compounds (pyrimidine or pyridazine rings). Many ferroelectric mixtures consisting of achiral LC’s with a smectic C phase and chiral dopand were prepared to improve the value of the spontaneous polarization123. In principle, all antiferroelectric lc’s show similar structures. 4-(1-methylheptyloxycarbonyl)pheyl-40- octyloxybiphenyl-4-carboxylate (MHPOBC) exhibits an antiferroelectric chiral smectic phase (Figure 41). The antiferroelectric compound with a similar structure was also synthesized and its physical properties investigated (Figure 42). The characteristic molecular and layer arrangement of the ferroelectric and antiferroelectric smectic CŁ phases in their unwound states are presented in Figure 43.
VI. APPLICATIONS OF LIQUID CRYSTALS
A. Current Display Technology
The most important application of liquid crystals are electro-optic devices, especially the various kinds of displays. After the invention of the simple twisted nematic display by Schadt and Helfrich124 in the beginning of the seventies, the development and subsequent commercialization in LCD technology increased in an incomparable way. Table 18125 demonstrates the triumphal procession of the liquid crystal based devices which was not possible without the tremendous research effort of chemists, physicists and electronic engineers. In the following we want to present the most important display devices and of course the materials which can be tailored for any aspired device property, e.g. nematic phase range, switching time, threshold voltage, contrast ratio and many more. Many of the compounds used contain the XDY unit described in the previous sections; especially, lc’s containing the CDO moiety were used in eutectic mixtures with ten or more components. Table 19 lists the requirements which modern displays have to fulfill126. Some of them like contrast ratio or display size, have been already realized with LC modules till now, while others like large viewing angle or brightness have not been established satisfactorily with the current technology. Even new competitors like coloured plasma displays in VGA (video graphics array: at least 640 ð 480 pixels, 16 colours or grey levels) resolution are available, and represent alternatives to the LCD technology127. In the following an overview about the actual display types is given:
TABLE 18. History of LCD development
Year |
Passive/Active device |
|
Application |
|
|
|
|
1971 |
invention twisted nematic (TN) cell |
|
|
1973 |
TN cell |
CMOS LCD calculator |
|
1975 |
TN cell |
electronic watches, and games |
|
1979 |
TN DOT matrix LCD |
alphanumeric displays |
|
1983 |
TN DOT matrix LCD |
graphical displays for pocket computers |
|
1986 |
super twisted nematic (STN) cell |
graphical displays for pocket computers |
|
1987 |
D-STN B/W display; TFT display |
laptop; 300 TV |
|
1988 |
colour D-STN display |
colour LCD camcorder |
|
1992 |
TFT display |
14,200 |
colour display |
1994 |
STN display |
10,400 |
SVGA colour display |
462 |
T. Hanemann and W. Haase |
||||||
|
TABLE 19. Profile of displays for multimedia application |
||||||
|
|
|
|
|
|
|
|
|
Item |
|
|
|
Characteristics |
||
|
|
|
|
|
|
|
|
|
Display dimension |
20 |
|
50 cm diagonal |
|||
|
|
||||||
|
Number of pixels |
106 |
|
|
|
|
|
|
DOT configuration |
256 grey scale or full colour |
|||||
|
Contrast ratio |
>100:1 |
|
|
|||
|
Viewing angle |
š90° |
2 |
|
|
||
|
Brightness |
1000 |
cd/m |
||||
|
Switching time |
0.1 ms |
|||||
|
Panel thickness |
0.1 cm |
|||||
|
Temperature range |
wider than 10 |
|
50 °C |
|||
|
|
||||||
|
Lifetime |
105 |
|
|
|
|
|
glass panel with ITO electrode and
orienting polyimide layer
polarizer
field off: bright |
field on: dark |
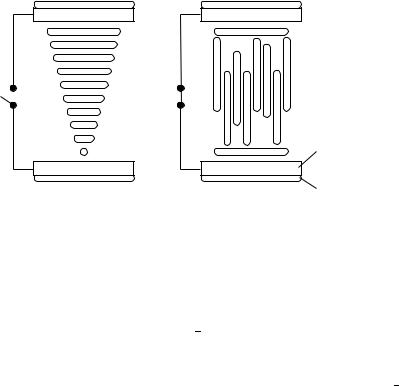
FIGURE 44. Schematic drawing of the TN cell
(1) Twisted Nematic (TN) Cell: The classical TN cell consists of two glass panels, which carry on the inside a conductive layer of indium-tin-oxide (ITO) as well as a polyimide layer in a sandwich construction. The polyimide acts as an aligning substrate and orients the director of the first nematic monolayer at the surface; the induced orientation at the top electrode is perpendicular to the one at the bottom but both are homogeneous in the plane of the surface. Typical characteristic properties of a commercial TN element are128 contrast: 20:1; driving voltage: 3 25 V; temp. range: 55 to C80 °C; switching time, on: 20 ms, off: 50 ms; viewing angle: š60° and a life-time of 105 h. Actual TN displays used in laptops allow for a realization of 16 or better 256 grey levels (standard VGA with 640 ð 480 pixel). Without an electric field and due to the nematic potential the alignment propagates through the whole liquid crystal layer (thickness between 2 10 m) (Figure 44, left side). According to the 90° twist of the orienting layers the director of the nematic phase turns from the top to the bottom electrode by 90° too. With the exception of the first lc monolayers all molecules orient parallel to the applied E field (Figure 44, right side). A thin polarizer foil is attached on top of each glass substrate, and the polarization is parallel to the orientation layer. In case of the field off, the linear polarization of the incoming light follows the twist of the molecules and the display occurs transparent. The polarization of the outcoming light is perpendicular to the incoming one. After switching on the E field, the twist of the nematic orientation is destroyed and the incoming light is blocked at the polarizer124,129.
