
9. Liquid crystals with XDY groups |
443 |
as mentioned above for the low molecular mass liquid crystals (Section IV). The only difference would be that the flexible terminal groups at one or both sides of the rigid mesogenic core possess another attachment at the opposite end either to a polymeric main chain, or to the next moiety, hence forming the chain themselves. A >CDX double bond can be incorporated into different parts of an LC macromolecule. The two most popular are (i) attachment groups between different structural parts of the molecule (main chain, spacers, mesogenic cores, terminal groups), and (ii) the bridging groups between aromatic rings within the mesogenic moiety. Side chain and combined LC polymers containing polymerizable vinyl groups (>CHDCH2) at the ‘free ends’ of the mesogenic side chains are also very important for the formation of crosslinked polymer networks.
From the very beginning of investigations on the LCP’s, the ester group was widely used in the chemical structure of all types of LCP’s. Thus, hundreds of the synthesized side chain LC polyacrylates and polymethacrylates contain an ester linkage between the main chain and the side groups49,50, as do also polychloroacrylates51 and many others. Side chain and combined polysiloxanes often have mesogenic cores attached to the spacers and/or to terminal hydrocarbon radicals by ester groups. Some LC polyacrylamides have been also synthesized52. According to the main chain LC polymers, the amide attachment is also very important, especially for the so-called lyotropic LCP’s which form the liquid crystalline phases in non-aqueous solutions. They are widely used in industrial production
O |
O |
( O |
O (CH2 )n ) |
O |
O |
O |
O |
O |
O |
(
(
O |
(CH2 )m |
O (CH2 )n ) |
|
NH |
NH |
O |
|
O |
X |
) |
|
|
|
|
O |
O |
|
O |
(CH2 )n |
O |
|
|
|
O |
O Cm H2 m +1 |
CH3 |
) |
|
|
( |
|
|
N |
|
|
|
|
|
NH |
NH |
N |
O |
(CH2 )n |
|
|
|
|
O |
|
X = H, Cl, CH3 ; m, n = 5 −11
FIGURE 20. Main and side chain LC polymers with ester and amide attachment groups
444
TABLE 12. LC behaviour and PS values for polymers with different attachment groups
Polymer Phase transition temp. (°C) PS (nC cm 2)
|
|
O |
|
(gl) 11 SFŁ 52 SCŁ 57 SA 116 I |
3.066 |
|||
|
|
|
|
|
O |
|
|
|
|
|
O |
(CH2 )10 O |
|
|
|
|
|
CH3 |
|
|
|
|
O |
|
|
|
O |
|
|
|
|
|
|||
Si |
|
|
|
|
(gl) 32 SCŁ 115 SA 123 I |
|
|
67 |
|
|
|
|
O |
|
|
||
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
(CH2 )8 |
O |
|
|
|
|
||
CH3 |
|
|
|
O |
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Si |
|
|
|
|
SHŁ 58 SCŁ 66 SA 170 I |
5658 |
||
|
|
|
|
|
||||
(CH2 )11 O
O
9. Liquid crystals with XDY groups |
445 |
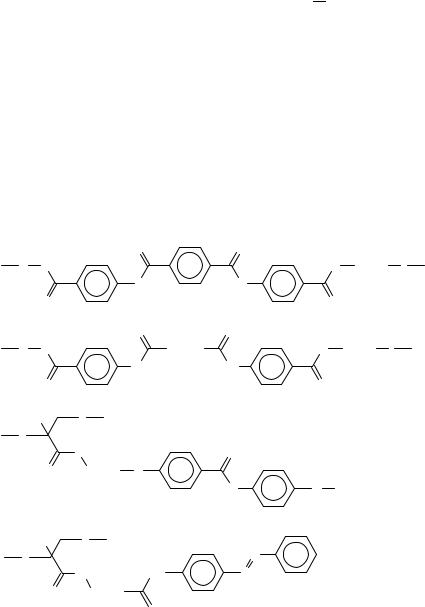
of high-strength construction materials and plastics (Kevlar, Vectra etc.). Figure 20 shows some examples of liquid crystals with ester and amide attachments53 56. Just recently some side chain polymers have been reported containing ketone attachment groups,C(DO) 57,58. As compared with the ester group, the ketone attachment results in higher clearing temperatures, i.e. in a wider range of LC behaviour. For the compounds forming the ferroelectric SmCŁ phase, the ketone bonding gives also higher maximum values of the spontaneous polarization PS. Table 12 presents the phase behaviour and PS values for some polymers with similar chemical structure of mesogenic side chains, but having different attachment groups between the biphenyl aromatic cores and the chiral terminal radicals.
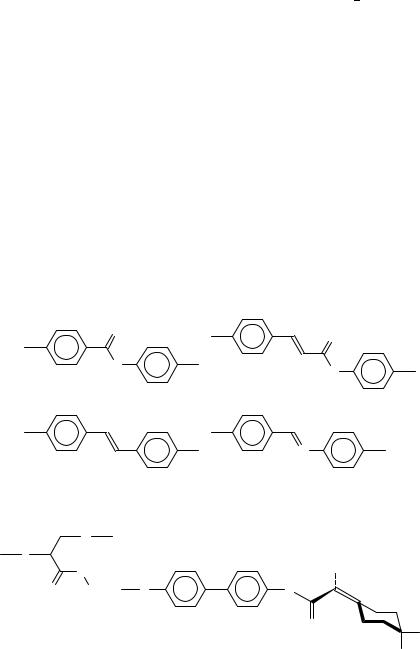
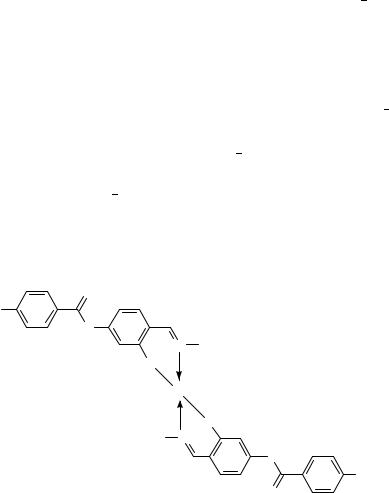
Many LC polymers contain double bonds in such mesogenic moieties as phenyl benzoates (A)59, phenyl cinnamoates (B)60, stilbenes (C)61 and benzylidenanilines (D)62 (Figure 21). Chirality is very important in the field of liquid crystals (cholesteric and ferroelectric smectic CŁ phases). Very interesting is the usage of double bonds to introduce the axial chirality in LC polymers, i.e. the absence of mirror symmetry not due to a single chiral carbon atom, but because of a large mesogenic fragment with lack of symmetry as a whole (Figure 22)63.
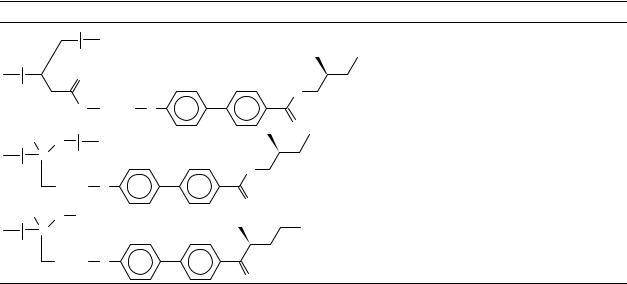
Crosslinked LC elastomers (Figure 19d) are very promising for piezoelectric and ferroelectric applications, and also as non-linear optic materials. The first synthetic step to such materials is the preparation of usual side chain or combined LC copolymers doped with a small part of side chains containing a polymerizable >CDC< double bond at the end (Figure 23 shows a particular example of a crosslinkable LC polymer64). The copolymer can be further photocrosslinked, giving an elastic polymer film which reveals
|
O |
O |
|
|
|
|
O |
O |
|
|
|
|
A |
B |
|
|
N |
|
C |
D |
FIGURE 21. Mesogenic aromatic cores used in side chain polymers |
||
( |
) |
|
|
H |
|
|
O |
|
|
|
|
O |
(CH2 )6 O |
O |
O
CH3
H
FIGURE 22. Side chain polymer with axial chirality

446 |
T. Hanemann and W. Haase |
O
|
|
|
|
|
|
O |
CH3 |
Si |
(CH2 )11 |
O |
O |
|
|
|
|
|
|
|
(CH2 )5 |
NH |
|
O |
|
|
|
|
0.1 |
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
CH3 |
Si |
(CH2 )11 |
O |
O |
Cl |
|
|
O |
|
|
O |
|
0.9 |
|
|
|
|
|
|
|
CH3 |
Si |
CH3 |
|
|
|
|
|
O |
2.7 |
|
|
|
|
|
|
|
|
|
|
FIGURE 23. Preparation of a crosslinked LC elastomer

9. Liquid crystals with XDY groups |
447 |
||
TABLE 13. Change in phase transitions after crosslinking the polymer |
|
|
|
|
|
|
|
Polymer |
Phase transition temp. (°C) |
||
Not crosslinked |
SX 29 SCŁ |
53 SA 89 I |
|
Crosslinked by 2 wt.% of init. and 365 nm UV radiation |
SCŁ 49 SA |
|
|
|
NO2 |
|
|
O |
O |
|
|
O |
|
|
|
O |
|
|
|
FIGURE 24. Main chain polymer synthesized via olefin metathesis reaction
ferroelectric switching and a piezoelectric coefficient up to 7 pC N 1. Phase transitions of the crosslinkable polymer and of the resulting LC elastomer are compared in Table 13. Before finishing this short review on liquid crystalline polymers containing >CDX double bonds, we should mention also the recent results of Walba and collaborators65 on the main chain LC polymers obtained by the olefin metathesis (Figure 24). The approach proposed seems to be very promising for the creation of advanced polymeric LC’s with CHDCH double bonds in the polymer chain.
B. Metallomesogenes
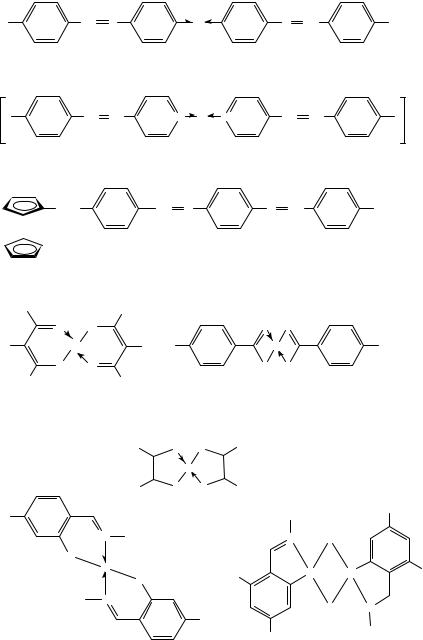
A rather new class of liquid crystalline materials combines mesomorphic properties with the remarkable magnetic and optical features of inorganic metal complexes, the so-called metallomesogenes. First we will start with a discussion of general aspects of metallomesogenes illustrated with compounds containing different XDY groups, and then we will proceed to the interesting magnetic properties of LC’s containing metal ions. For a more detailed description of this essentially interdisciplinary topic, the reader is strongly referred to the excellent reviews written by Espinet’s group68, Hudson and Maitlis69 or GiroudGodquin and Maitlis70. The de novo design of a metal complex exhibiting mesomorphic behaviour is based on the sophisticated choice of appropriate ligands. The chemical classification proposed in Table 2 (Section IV) presents a wide variety of different functional groups which are common in pure organic liquid crystals. Hence typical metallomesogenic representatives are given in Figure 25. Some of these molecular moieties can in principle be coordinated to metal ions and therefore act as donor groups in ligands suitable for the formation of metal-containing LC’s. In contrast, only a limited number of ligand types have tended to dominate the field of rod-like LC’s, including the following:
žmonodentate ligands: 4-stilbazoles (or azomethines) and 4-substituted pyridines (A,B),
žbidentate chelate moieties: most of them contain carbonyl-type groups such as 1,3-diketones, malondialdehydes (D), carbonic acids, azomethines (G) and sulphur analogues ( CDS) such as thiocarbonic acids (E), 1,3-dithiolenes (F) orthometallated imines (H) and azines,
žmiscellaneous ligands like ferrocenes71 (C), all in Figure 25.
Following the general scheme of the basic structural elements of rod-like liquid crystalline molecules, the donor atom or set of donor atoms can be
448 |
|
|
T. Hanemann and W. Haase |
|
|
||
R |
CH |
N |
|
M |
N |
CH |
R |
|
|
|
|
A |
|
|
|
|
|
|
|
|
|
|
n+ |
R |
CH |
CH |
N |
M N |
CH |
CH |
R |
|
|
|
|
B |
|
|
|
|
COO |
|
CH |
N |
N CH |
|
R |
|
Fe |
|
|
|
|
|
|
C
R1
R2
R1
R2
FIGURE 25.
|
R1 |
|
|
|
|
|
|
O |
O |
|
|
S |
S |
|
|
M |
R2 |
R |
|
M |
|
|
R |
|
|
|
|
||||
O |
O |
|
|
S |
S |
|
|
|
R1 |
|
|
|
|
|
|
D |
|
|
|
E |
|
|
|
|
R2 |
S |
S |
R1 |
|
|
|
|
|
|
|
|
|
||
|
|
M |
|
|
|
|
|
|
R1 |
S |
S |
R2 |
|
|
|
|
|
F |
|
|
R1 |
|
R2 |
|
|
|
|
|
|
||
|
N R1 |
|
|
|
N |
Y |
|
O |
|
|
|
|
|
|
|
|
M |
|
|
X |
M |
M |
X |
|
O |
|
|
||||
|
|
|
|
|
|
|
|
R1 |
N |
|
|
|
|
Y |
N |
|
|
|
R2 |
|
|
||
|
|
|
|
|
|
R1 |
|
|
|
|
|
R2 |
|
|
|
|
|
|
|
|
|
|
|
|
G |
|
|
|
|
H |
|
Structures of representative examples of metallomesogenes

9. Liquid crystals with XDY groups |
449 |
žlocated in the rigid ring system (A,B,H),
žlocated in the central middle group (G, H with R1 being aliphatic and D with R1 and R2 being aromatic),
žattached on the aliphatic chains (C),
žattached as a polar group to the central rigid ring system (G, H with R1 being aromatic, D with R1 being aromatic and R2 being aliphatic or vice versa) and, as shown in E and F, all in Figure 25.
The phase formation must be regarded as a consequence of the overall shape of the mesogenic molecule. Consequently, the complexation with long monodentate ligands such as aryls or 4-stilbazoles leads to nematic or smectic phases. It must be pointed out that Hg-
aryls (Figure 25A with M D Hg) |
|
presented long ago in the year 1910 |
|
can be regarded |
||
|
|
|||||
|
|
72 |
. Thirteen years later, Vorlander¨ reported that |
|||
as the first example of a metallomesogen |
|
|||||
diarylmercury compounds form smectic phases73. Since substituted pyridines are able to form stable complexes with different metal ions, they are often used for the synthesis of metallomesogenes. Bruce and coworkers74 reported on Ag(II) bis-stilbazole compounds, and showed that the reaction of Ag salts with stilbazoles lead to the first ionic mesogenes with high transition temperatures. The polymorphism of such compounds may be interesting for possible applications in electrochemistry. The first metal-containing liquid crystalline compound based on a ˇ-diketone ligand was obtained by the complexation of Pd(II) with a complex having R1 D H; R2 D Ph-OC8H17 (Figure 25D)75. The general structure of ˇ-diketones (R1 D alkyl, R2 D H) or malondialdehydes (R1 D H and R2 D alkyl) used for the synthesis of metallomesogenes is displayed in Figure 25D. ˇ- Diketones are able to form square-planar complexes with a wide varity of metal ions [Ni(II), Pd(II), Pt(II), Cu(II)]. Complexes with R1 D alkyl and R2 D H form discotic phases76,77 whereas compounds having the inverted substitution pattern (R1 D H and R2 D alkyl) tend to form nematic or smectic phases78. In general, rod-like ligands with alkyl chains extended parallel to each other give rise to calamitic crystals whereas disc-like ligands tend to form discotic phases.
Some examples have promising magnetic properties, but for possible applications it is necessary to increase the chemical and thermal stability of the metal containing LC’s. It is known that the complexation of d-metal ions with sulphur-containing ligands leads to extremely stable complexes. Hence a large number of metallomesogenes containing CDS groups were synthesized. The synthesis and phase characterization of dithiolen complexes (Figure 25F) was reported by Giroud-Godquin and coworkers79,80. The X-ray structure investigation on single crystals of a compound with R2 D H and R1 D Ph-C8H17 with M D Ni(II) showed that in the crystalline phase the phenyl groups in the chains were arranged in trans-position81. Complexes of Ni(II) and Pd(II) with dithioacids (for general chemical structure, see Figure 25E) and long alkyl chains82 form smectic phases; the presence of shorter chains results in a nematic phase70. Orthometallated imines (Figure 25G) have been synthesized by Barbera and collaborators83. The dinuclear M D Pd(II) complexes with Y D Br, Cl, SCN and Y D H, Cl, Br, CN exhibit smectic phases, whereas in the case of Y D OAc , the geometry of the bis-acetate bridging unit Pd (OAc)2 Pd is assumed to cause a drastic deviation from the planar arrangement of all phenyl groups and, as a consequence, no mesogenic behaviour was observed.
One important reason for the current research is the combination of molecular ordering due to the anisotropic physicochemical properties, and the fact that many metal ions possess unpaired electrons and, as a consequence, interesting magnetic properties arise. Selected examples of magnetic investigations on azomethine complexes are given
450 |
T. Hanemann and W. Haase |
in the following: The electronic ground state of Ni(II) complexes correlates strongly with their coordination, consequently variations in the coordination which may occur at the crystalline ! mesophase or mesophase ! isotropic phase transition can result in drastic changes of the magnetic or optical properties. In case of mesogenic Ni(II) Schiffbases (for general chemical structure, see Figure 26) it was reported that a conversion of the total spin state of their metal ion as a consequence of a variation in coordination geometry from planar to tetrahedral takes place at the phase transition from crystalline to mesogenic84. The reported magnetochromism can be considered as molecular bistability. Therefore, such a kind of liquid crystalline compound may play an important role in the emerging field of molecular electronics, i.e. the use of molecular systems in electronic circuits and devices. The short-range order in a mesogenic phase may provide the existence of magnetically ordered structures in metallomesogenes. EPR investigations on a Cu(II) ˇ- diketone complex85 provided evidence for the existence of a one-dimensional magnetic ordered structure in the crystalline phase. Similar results obtained from magnetic susceptibility measurements have been presented for a Cu(II) malondialdehyde complex74. In addition, using polynuclear clusters enables the design of special intramolecular exchange interactions. Such a kind of antiferromagnetic exchange interactions has been reported for a dinuclear oxo-bridged Fe(III) azomethine complex86. The incorporation of a metal ion possessing a large paramagnetic anisotropy enhances the overall magnetic anisotropy and leads to liquid crystalline compounds which can be easily oriented in an external magnetic field. This has been shown in the case of some rare-earth-containing azomethine compounds87.
O
R′
O
N R
O
Ni
O
R N
O
R′
O
R′ = |
|
OC H |
R = |
|
CH C*H(CH )C H |
||||||
|
|
||||||||||
|
|
7 |
15 |
|
|
2 |
3 |
2 |
5 |
||
R′ = |
|
|
OC12 H2 5 |
R = |
|
CH2 C*H(CH3 )C2 H5 |
|||||
|
|
|
|||||||||
R′ = |
|
OC H |
R = |
|
C*H(CH )C H |
|
|||||
|
|
|
|||||||||
|
|
7 |
15 |
|
|
3 |
11 |
2 3 |
|
||
R′ = |
|
|
OC H |
R = |
|
|
C*H(CH )C H |
|
|||
|
|
|
|
|
|||||||
|
|
7 |
15 |
|
|
3 |
6 |
13 |
|
||
FIGURE 26. Structure of the mesogenic Ni(II) complexes exhibiting magnetochromism

9. Liquid crystals with XDY groups |
451 |
C. Carbohydrate Liquid Crystals
With carbohydrates, these transition temperatures are sensitive to the rates of heating and cooling, to the history of the sample, and due to the impurities by decomposition products, for example. For these reasons, they may not be exactly reproducible between samples and investigators. G. A. Jeffrey88
For these the clearing points during heating and cooling show considerable differences (5 7 °C). We have never observed this phenomenon in carbohydrate mesogenes before, and can offer no explanation this time. H. A. van Doren and coworkers89
The above statements of two of the most important scientists in this research area characterize a real serious problem, the reproducibility of the experimental results. Nevertheless, outstanding reviews about carbohydrate LC’s are available in the literature and strongly recommended for further reading88,90. The basic structure element of all cyclic carbohydrates is an XDY moiety as a terminal or inner carbonyl group and a hydroxy group of an aliphatic polyalcohol forming a cyclic hemiacetal, usually a furan or pyran derivative (Figures 27 and 28). Though liquid crystalline carbohydrates (LC-CH) have been known for several decades, they still have the status of a more exotic class of organic materials. In 1911, E. Fischer observed the double melting behaviour at a series of long-tailed alkyl-pyranosides91, which were identified in 1938 as thermotropic liquid crystals92. Even in 1967 scientists still described mesomorphism of similar compounds only as a double melting behaviour (Figure 29a)93. Until the mid-eighties scientific interest was very small, and by 1991 approximately 1000 LC-CH’s with mesomorphic properties had been described in the literature. The first thioglycosides were synthesized in 1982 (Figure 29b)94, the first chiral columnar discotic phase of a peralkylated glucopyranose was observed in 1985, and in the same year the boundary between thermotropics and lyotropics mentioned in Section II blurred (Figure 30a)95 97, the given SAd phase in n- decyl-ˇ-D-glucopyranoside was shown to be identical with the lyotropic one containing up to 32% water (Figure 30b). As a result of the rapid increase new kinds of different
O |
H |
|
CH2 OH |
|||
|
|
|
||||
HO |
OH |
HO |
|
|
|
O |
|
|
|
||||
|
|
|
||||
OH |
|
|
|
OH |
||
|
|
|
||||
|
|
|
|
|
||
|
|
|
|
|
||
|
OH |
|
|
|
|
OH |
|
|
|
|
|
||
|
CH2 OH |
|
|
|
CH2 OH |
|
|
|
|||||
FIGURE 27. D-Glucose and D-fructose as typical carbohydrate representatives
|
OH |
|
OH |
|
|
|
|
|
|
|
O |
HO |
|
OH |
|
|
|
|
|
HO |
|
OH |
|
|
HO |
|
HO |
|
|
|
OH |
O |
OH |
|
|
|
FIGURE 28. Chair conformation and pyrane description of ˇ-D-glucopyranose
452 |
|
T. Hanemann and W. Haase |
|
||
|
OH |
|
|
|
|
|
O |
OH |
|
OH |
|
HO |
|
|
|
||
HO |
|
|
|
OH |
|
|
OH |
O |
|
O |
|
|
O |
|
OC14 H2 9 |
HO |
SC7H15 |
|
HO |
|
|
HO |
|
|
|
OH |
|
|
|
|
(a) |
|
|
(b) |
|
|
C 106 ? 258 I |
|
C 64 N 151 I |
(°C) |
|
FIGURE 29. |
(a) 1-Tetradecyl-4-O-(˛-D-glucopyranosyl)-ˇ-D-glucopyranoside and (b) 1-heptyl-thio-˛- |
||||
D-mannopyranoside |
|
|
|
|
|
|
OC10 H21 |
|
|
OH |
|
|
O |
|
|
O |
|
H21C10 O |
|
|
|
||
|
HO |
OC10 H21 |
|||
H21C10 O |
|
||||
OC10 H21 |
|
HO |
|
||
|
OC10 H21 |
|
OH |
|
|
|
(a) |
|
|
(b) |
|
|
|
|
|
|
|
|
C 27 (D 33) I |
|
|
C1 65 C2 70 SAd 134 I (°C) |
|
|
C 31 (D 37) I |
|
|
|
|
FIGURE 30. (a) Penta-O-decanoyl-(˛/ˇ)-D-glucopyranoside and (b) 1-decyl-O-ˇ-glucopyranoside
LC-CH classes were investigated, N-substituted aldonamides showing a lamellar phase without solvent were synthesized98,99 (Figure 31a) followed by n-alkyl-gluconamides in 1986 (Figure 31b)100 and different alkylamidoglucitols (Figure 31c)101 as well as the first dithioacetals (Figure 31d)102. The mesophase of the latter was first identified to be smectic, but later revised to be discotic hexagonal103. Like in common LC’s, carbohydrates with short aliphatic chains (n < 6) do not normally possess any mesophase, and in mid-sized chain lengths (n D 6,7) only monotropic ones could be observed. Extended chains (n > 8) lead to mesomorphism, mostly identified as smectic phases. The very interesting family of discotic liquid crystals were accessible at the end of the eighties and beginning of the early nineties: a columnar hexagonal phase was identified via X-ray diffraction103, the first dior oligosaccharides with columnar discotic arrangement in 1991104 as well as the important group of acylated glycosides in the same year105 (Figure 32). In the last ten years carbohydrate LC’s with nearly every kind of typical sugar moiety were synthesized, but in the following we will concentrate on the various kinds of glycosides. In a large number of systems it is possible to establish a structure-mesomorphism relationship. Additionally, well accepted models for the molecular arrangement in the widely distributed SA phase will be presented.
An excellent overview dealing with liquid crystalline glycosides containing cyclic carbohydrate portions was written by Vill’s group106; Goodby and coworkers101 presented a
