
9. Liquid crystals with XDY groups |
433 |
is preferred like in other series. The more complex and space-filling tris-benzoate shows a monotropic SB and an enantiotropic SA phase. The large molecular mass of one single molecule causes the phase transition temperatures to an occasional slight change with increasing chain length. Similar molecules like the above-mentioned ones often possess discotic phases, i.e. the molecular shape is disc-like or closely related; different phase types like discotic nematic, or columns with hexatic arrangement, can be found. The molecule on the left-hand side in Figure 7 is close to that presented in the previous figure, but the resulting mesomorphic behaviour taken from Table 4 is totally different. A similar disc, but derived from a more complex aromatic system, is drawn on the right-hand side of Figure 7, the hexa-alkanoates of triphenylene. Solid state polymorphism as well as one discotic mesophase occur in the first molecule; with increasing chain length the phase width decreases till the mesophase finally disappears. In the triphenylene-derived hexa-alkanoates three different columnar discotic phases can be observed, while longer chains result in the depression of all transition temperatures. Introduction of an alkylated benzoate unit in the triphenylene series leads to an exotic group of materials with mesomorphic properties, the carbonaceous liquid crystals. Thermal treatment of organic, especially polyaromatic, molecules results in a graphitization and large, planar compounds were formed21. Besides the columnar discotic phase a new fluid phase with low ordering was observed; due to the similarity of the great fluidity and the same schlieren texture the phase was labelled discotic nematic ND (Table 4c). Short aliphatic wings allow for high-temperature mesophases; elongation again decreases all transition temperatures21.
B. Liquid Crystals Containing Azo-, Azomethine, Stilbene or Tolane Units
At the beginning of the seventies and early eighties aspired material properties with regard to display applications were the only driving force for the search and synthesis of new lc compounds. However, later on new and promising research topics appeared, so we concentrate here on additional molecular quantities essential for applications in the wide area of non-linear optics22 25. In addition to the common building blocks for lc molecules, polar groups with opposite electron affinity, like dialkylamino and nitro, must be attached at an elongated conjugated -system (push pull system) containing aromatic rings as well as bridging groups like azo, stilbene, tolane or azomethine (Figure 8a). The latter interrupts the conjugated system due to the dihedral angle upto 60° between the substituents attached at the azomethine bond. Simple lc’s containing a stilbene unit were described by Fouquey and coworkers26 (see Figure 8b and Table 5). The mesomorphism
A ( |
|
|
|
R1 |
X |
|
|
N |
|
|
Y ) |
D |
O2 N |
2 |
|
|
R |
||
|
n |
|
|
|
|
(a) |
|
|
(b) |
FIGURE 8. (a) Basic structure of an organic molecule with non-linear optical properties (A: acceptor, D: donor unit) and (b) liquid crystalline stilbenes
TABLE 5. Mesomorphic properties of simple stilbene liquid crystals
|
Compound |
|
|
Phase sequence (°C) |
|||
1 |
D C12H25; R |
2 |
D H |
|
|
C 109.0 SE |
141.0 I |
R1 |
|
2 |
|
||||
R1 |
D C7H15O p C6H4CH3; R |
|
2D H |
C (94.0) SE |
100.0 I |
||
R |
D C12H25O p C6H4CH3; R |
D H |
C1 99.0 C2 |
108.0 SA 178.0 I |
|||
434 |
T. Hanemann and W. Haase |
has been introduced using elongated chains as wing end groups, when due to the extended total molecular length ordered smectic phases are favourable. The presence of aromatic moieties in the side chain suppresses these ordered structures and leads to monotropic phases or to solid state polymorphism combined with a simple SA phase. The given push pull system should result in improved non-linear optical properties. With increasing molecular length and enhanced structural complexity a more and more complicated phase behaviour is expected. The addition of a second bridging group to the azobenzene, stilbene or azomethine core results in the appearance of a large number of different phases. Figure 9 shows ten substituted azomethines with either a stilbene or a tolane core;
R
N
O2 N
ST-
R
O2 N |
N |
ΤΟ-
O
O
R: O
O
AZM-A |
AZM-Β |
O
OH
AZM-C
N
HO
AZM-D |
AZM-Ε |
FIGURE 9. Structures of stilbene and tolane derived azomethine liquid crystals

|
9. Liquid crystals with XDY groups |
435 |
|
TABLE 6. Mesomorphic properties of azomethine/stilbene/tolane liquid crystals |
|||
|
|
|
|
Compound |
Phase sequence (°C) |
Trans. enthalpy (kJ mol 1) |
|
ST-AZM-A |
C 177.3 N 259.3 I |
|
26.3/0.2 |
ST-AZM-B |
1. cycle: C1 99.3 SE |
128.3 SB 132.6 SA 164.2 |
22.3/2.2/7.9/n.a./0.2 |
|
N 181.9 I |
|
|
|
Change in the 2. cycle: |
|
|
|
C1 96.6 SE; C2 |
116.1 SE |
|
ST-AZM-C |
C 154.9 SE 165.5 SB |
174.6 N 300.0 decomp. |
22.3/1.3/5.9 |
ST-AZM-D |
C 300.0 decomp. |
|
n.a |
ST-AZM-E |
C 307.0 decomp. |
|
n.a |
TO-AZM-A |
C 194.4 N 212.6 I |
|
39.9/0.2 |
TO-AZM-B |
C 221.6 N 300<I |
|
15.8/n.a |
TO-AZM-C |
C 139.1 SX 152.4 275.5 I |
22.3/5.1/0.3 |
|
TO-AZM-D |
C 233.5 I |
|
n.a |
TO-AZM-E |
C 263.9 N 298.4 I |
|
30.0/0.2 |
|
|
|
|
TABLE 7. Structural anisotropy of stilbene and tolane based azomethines
ST/TO-AZM-A ST/TO-AZM-B ST/TO-AZM-C ST/TO-AZM-D ST/TO-AZM-E
Length/width |
2.2/2.6 |
2.2/2.9 |
2.9/4.5 |
1.7/2.1 |
2.3/2.8 |
|
0.21/0.16 |
0.21/0.14 |
0.05/0.13 |
0.41/0.26 |
0.22/0.08 |
|
|
|
|
|
|
Table 6 compares the mesomorphic properties27 31. In case of compound TO-AZM-B the unprotected aldehyde was unfortunately obtained, even when we started with the diethoxyprotected one. The presence of extended aliphatic chains are not an imperative necessity for the introduction of liquid crystallinity; even short polar groups allow for mesomorpic behaviour. With the exception of the naphthyl compound all molecules show a pronounced structural anisotropy numerically expressable either using the length/width ratio or using the more sophisticated structural biaxiality parameter 32 with the long molecular axis as the principal axis z, and x and y as the short ones (equation 1). Important for the estimation of is the difference between the two short axes relative to the molecular length. An enhanced uniaxial structural anisotropy yields a small -value, 40 -pentyl-4-cyanobiphenyl (CB5) as a typical nematic liquid crystal, which has an estimated length-to-width ratio of 2.6 and a biaxiality parameter of 0.01. Hence the values given in Table 7 for stilbene and tolane differ slightly due to the various XDY linkage groups.
p |
|
|
y x |
|
|
||||
D 0.25 ð 6 ð |
|
1 |
||
|
||||
|
|
|
z 0.5y C x |
|
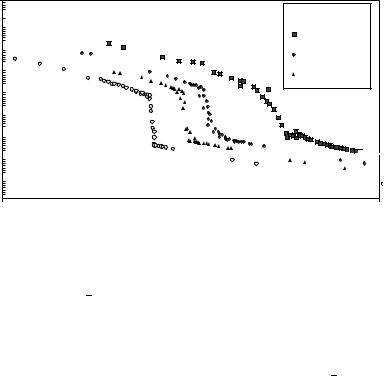
Generally speaking, the tolanes possess an improved structural anisotropy in comparison to the stilbenes, hence mesogenic phases with low ordering are preferred; TO-AZM-E shows liquid crystalline behaviour opposite to that of ST-AZM-E, while both naphthalene compounds exhibit only normal melting. Mixtures of the mentioned stilbene/azomethines with simple nematics like CB5 are interesting for coloured guest host displays because, due to the pronounced structural anisotropy, they stabilize the nematic phase even at room temperature far beyond their own temperature range of liquid crystallinity. Normally, common solutes like solvent molecules or anthraquinone dyes act as ‘impurities’ and disturb the nematic phase and depress the clearing temperature. High-precision density measurements can be used as a reliable method for the determination of the clearing point due to the density gap between the nematic and the isotropic phase30. Figure 10 shows the change in density of the mixtures at three different concentrations (mole fraction
436 |
T. Hanemann and W. Haase |
d (kg/m3)
1.015

 CB5 1.014
CB5 1.014 


CB5/AZM-C3
1.013 
CB5/AZM-C2
1.012 
CB5/AZM-C1
1.011 
1.010 
1.009 
1.008 










 1.007
1.007  1.006
1.006 



































































































0.996 |
0.997 |
0.998 |
0.999 |
1.000 |
1.001 |
1.002 |
1.003 |
1.004 |
1.005 |
1.006 |
|
|
|
|
|
T* |
|
|
|
|
|
FIGURE 10. Reduced temp. vs density plot at different concentrations of ST-AZM-C in CB5
x ð 103 D 0.6; 1.1; 2.3) of the solved dye in the nematic host CB5. Increasing dye amount raises the nematic isotropic transition temperatures T D 0.40, 0.69, 1.55 °C relative to the value of pure CB5, 34.85 °C, defined as reduced temperature TŁ D 1. Additionally, the order parameter Sop as a measure of the nematic ordering estimated via UV/Vis dichroism experiments is elevated with increasing dye amount. Pure CB5 possesses an order parameter around 0.44 (TŁ D 0.999)33; the investigated mixtures all show an improved value (0.52, 0.55, 0.56) at the same reduced temperature. As a consequence the accessible contrast ratio between onand off-state in a guest host display should be improved too. For these elongated molecules with two linkage groups the optical order parameter can be predicted within a certain range using easily accessible thermodynamic data30.
Another series of molecules containing one or two XDY moieties are presented in Figure 1134. The two simple stilbenes ODAPS and ODANS were forced to be liquid crystalline via the octadecyl group; due to the missing polar endgroup the pyridyl compound shows only a monotropic phase and solid state polymorphism (Table 8). The introduction of the nitro group results in a more complex phase behaviour; again an ordered SE phase can be observed. The other molecules differ basically in the bridging groups between the aromatic portions. DEANBAZB with the rigid core, the small diethylamino group and the resulting enhanced shape anisotropy has higher transition temperatures comparable to the related azomethine compounds presented earlier. The substitution of one azo link by a stilbene unit and the hexyl groups lowers the phase-transition temperatures drastically; in the related molecule 40-N,N-dihexylamino-4-nitrostilbene with only one bridging group, no mesomorphism occurs. Further substitution and a lateral cyano moiety leads to a monotropic phase. Another group of molecules with lc as well as potential non-linear optical properties contains a cinnamoyl moiety which can be photocrosslinked (Figures 12 and 13). In both cases a nematic phase occurs and again the mesophase of the nitro compound ZSNAZB is located at higher temperatures and is stable till decomposition around 300 °C35,36. The presence of the flexible butyl group dramatically lowers the phase-transition temperatures (Table 9). The purpose of the synthesis was the affixation of the dyes using UV-light at a modified polymethacrylate containing a cinnamoyl moiety in the side chain too and the formation of an additional network. The application of
9. Liquid crystals with XDY groups |
437 |
NHC18 H3 7
N
ODAPS
N |
N(C6 H13 )2 |
N
O2 N
DHANSAB
NHC18 H3 7
O2 N
ODANS
N(C6 H13 )2
O2 N
NC
DHACNNBS
N |
N(C6 H13 )2 |
N N
O2 N |
N |
DEANBAZB
FIGURE 11. Structures of stilbene and azo derived liquid crystals
photocrosslinking of cinnamoyl-substituted compounds and the resulting formation of a discotic mesophase has been described37.
Liquid crystals with a large molecular mass are able to form a glassy state with mesomorphic behaviour. Yitzchaik and coworkers38 report about a new class of copolymers with non-linear optical properties, and a monomeric representative with a glassy metastable
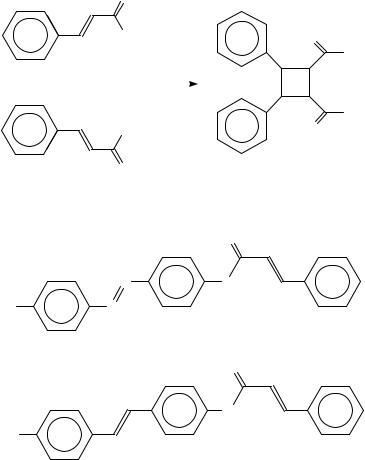
438 |
|
T. Hanemann and W. Haase |
|
|||||
TABLE 8. Polymorphic properties of stilbene and azo derived compounds |
|
|||||||
|
|
|
|
|
|
|
||
Compound |
|
Phase sequence (°C) |
|
Trans. enthalpy (kJ mol 1) |
||||
ODAPS |
1. |
cycle, heating: C1 |
70.0 C2 |
83.8 C3 |
99.6 I |
2.6/51.0/16.2 |
||
|
|
cooling: C |
87.8 SC |
97.0 I |
|
22.9/7.3 |
||
|
2. |
cycle, heating: C 95.8 I |
|
|
|
38.6 |
||
ODANS |
C 110.6 SE 129.1 SC |
135.2 I |
|
|
|
52.1/6.6/2.2 |
||
DEANBAZB |
C 222.8 SX 260.6 I |
|
|
|
|
|
25.9/6.7 |
|
DHANSAB |
C 172.6 SC 181.1 I |
|
|
|
|
|
25.7/3.6 |
|
DHACNNBS |
Heating: C 137.6 I |
|
|
|
|
|
31.0 |
|
|
Cooling: C 118.7 SE |
137.6 I |
|
|
|
27.0/2.5 |
||
|
|
O |
|
|
|
|
|
|
|
|
OR |
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
OR |
|
|
|
|
313 nm |
|
|
||
|
|
|
|
|
|
|
|
OR |
|
|
OR |
|
|
|
|
|
O |
|
|
O |
|
|
|
|
|
|
FIGURE 12. UV-induced crosslinking of cinnamic acid derivatives |
|
|||||||
|
|
|
|
|
|
|
O |
|
|
|
N |
|
|
|
|
O |
|
O2 N |
|
N |
|
|
|
|
|
|
ZSNAZB
O
NH
H9 C4
ZSBST
FIGURE 13. Structures of stilbene and azo derived cinnamic acid compounds
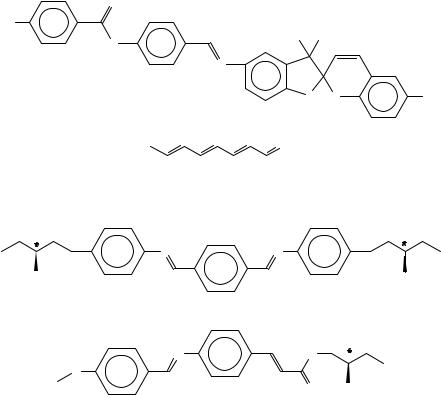
9. Liquid crystals with XDY groups |
439 |
||
TABLE 9. Polymorphic properties of stilbene and azo |
|
||
derived cinnamic acid derivatives |
|
|
|
|
|
|
|
Compound |
Phase sequence (°C) |
|
|
ZSNAZB |
C 268.7 N 318.0 |
(decomp.) |
|
ZSBST |
C 115.4 N 184.3 |
I |
|
|
|
|
|
O
NC
O
N
N O |
NO2 |
(a)
NR
(b)
FIGURE 14. (a) Spiropyran with glassy nematic state and (b) polyene azomethine liquid crystal
N N
TBMPA
N O
O
O
MMBC
FIGURE 15. Chiral nematics with blue phases41,46
nematic-like mesophase can be depicted from Figure 14a39. The simplest azomethine lc contains only one polyene group (as the rigid part) and an aliphatic chain on both sides of the azomethine bond (Figure 14b). R varies from methyl to n-decyl, and all of them show smectic properties, the latter one only a monotropic mesophase. With increasing alkyl chain lengths the clearing points decrease slightly from 78 to 63 °C; the melting points rise from 35 to 75 °C40.
A different phenomenon is the appearance of the so-called blue phases in case of chiral LC’s, first observed at cholesteric LC’s. Sometimes, one or more blue phases occur close below the clearing point with a very small phase width and bright blue or green colours. Figure 15 shows some typical representatives containing one or more XDY groups in
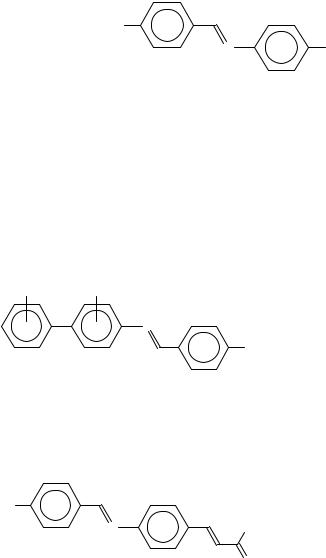
440 |
|
|
|
T. Hanemann and W. Haase |
|
|
|
|
|
|||||||
|
|
CnH2 n+1O |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
N |
|
|
|
Cm H2 m +1 |
|
|
||||
FIGURE 16. |
Structure of the N-(4-n-alkoxybenzylidene)-40 -n-alkylanilines (nO.m) |
|
|
|||||||||||||
|
TABLE 10. Transition temperatures (°C) of the 6O.m series42 |
|
|
|
|
|
||||||||||
|
M |
m.p. |
SG-SB or SA |
SB-SA- or N |
SG-N |
SA-N |
N-I |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
58 |
(44) |
(53) |
|
|
|
|
|
|
76 |
|
|||||
|
|
|
|
|
|
|
||||||||||
2 |
47 |
|
|
|
|
|
|
58 |
|
|
|
70 |
|
|||
|
|
|
|
|
|
|
|
|
|
|||||||
3 |
34 |
61 |
|
|
|
|
|
|
68 |
|
81 |
|
||||
|
|
|
|
|
|
|
|
|||||||||
4 |
30 |
57.5 |
59 |
|
|
|
69.5 |
78 |
|
|||||||
|
|
|
|
|||||||||||||
5 |
40 |
45 |
62.5 |
|
|
|
75 |
|
85 |
|
||||||
|
|
|
|
|
||||||||||||
6 |
15 |
35 |
63 |
|
|
|
77 |
|
82 |
|
||||||
|
|
|
|
|
||||||||||||
74 |
27 |
|
|
|
66 |
|
|
|
80.5 |
84 |
|
|||||
|
|
|
|
|
|
|
||||||||||
8 |
29 |
|
|
|
66.5 |
|
|
|
81.5 |
83 |
|
|||||
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3′X 2′ |
2 X |
3 |
|
|
|
|
|
X TNl (°C) ∆TNl (H - >Cl) (°C) |
||||||||
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
H |
163.5 |
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
OC7H15 |
3 Cl |
96.0 |
|
|
67.5 |
|
||
|
|
|
|
|
|
|
|
|
2′ Cl |
52.0 |
|
|
111.5 |
|
||
|
|
|
|
|
|
|
|
|
2 Cl |
45.0 |
|
|
118.5 |
|
||
|
|
|
|
|
|
|
|
|
3′ Cl |
smectic |
− |
|||||
|
|
|
|
|
|
|
|
|
alkyl |
TN l (°C) |
||||
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
CH2 CH2 CH2 CH2 CH3 |
136.5 |
||||||||
|
|
|
|
|
||||||||||
NC |
|
|
|
|
|
|
CHCH2 CH2 CH2 CH3 |
<20 |
||||||
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
||||||||
|
|
|
|
|
|
|
||||||||
N |
OR |
|
|
|
CH2 CHCH2 CH2 CH3 |
112 |
||||||||
|
O |
|
|
|
||||||||||
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
CH3 |
|
||||||
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
CH2 CH2 |
|
CHCH2 CH3 |
103 |
|||||
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
||
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
CH2 CH2 CH2 |
|
CHCH3 |
119.5 |
|||||
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
FIGURE 17. Top: Lateral substituted biphenyls, bottom: chain branched cinnamoyl liquid crystals
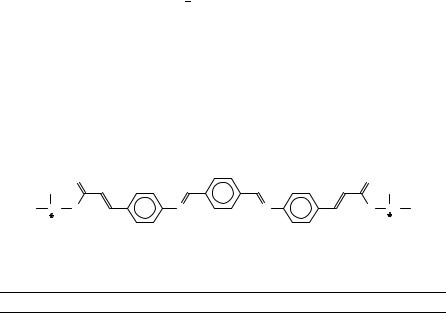
9. Liquid crystals with XDY groups |
441 |
chiral nematics. The first compound (TBMPA) has the following phase sequence41:
C 91 SX1 133 SX2 160 NŁ 169 BP 170 I [°C].
Following the simple azomethines as mentioned before, comprehensive studies on N-(4-n-alkoxybenzylidene)-40-n-alkylanilines (Figure 16) allow for a general structuremesomorphic property investigation. Variations in the two chains lengths and the azomethine unit (CDN or NDC) are both possible, but the authors concentrated on the first item. As typical representatives, Table 10 lists the transition temperatures of the 6O.m series. Shorter chains favour the formation of the SG phase, and in general complex phase sequences appear, while a weak odd even effect for the clearing point can be observed, and the change of the melting point seems to be occasional. More data with respect to different chain lengths were reported by Goodby and collaborators42.
Another interesting structure property relationship can be depicted from the influence of lateral substituents (Figure 17,top) or chain branching (Figure 17,bottom)43. In the first one a broadening of the molecule influences the phase behaviour; the lateral substituents in the 2 or 20 positions reinforce the twist between the phenyl groups and decrease the clearing point by about 120 °C. The branching of the terminal aliphatic wing group strongly affects the transition temperatures if the branching is far apart from the free chain end. The last-mentioned example is related to a quite new research topic, the antiferroelectric liquid crystals, which are very interesting for new and fast lc display.
O |
|
|
O |
|
CH3 |
|
|
|
CH3 |
H2 n+1Cn CH O |
N |
N |
O |
CH CnH2 n+1 |
FIGURE 18. Structure of 1-methyl-alkyl-terephthalidene-bis-aminocinnamate (MnTAC)
TABLE 11. Polymorphism of (a) chiral and (b) racemic 1-methyl-alkyl-terephthalidene-bis-aminocin- namate
(a) Chiral 1-methyl-alkyl-terephthalidene-bis-aminocinnamate
n |
Compound |
C |
|
SOŁ |
|
|
|
SQ |
|
SCŁ |
|
SA |
|
I |
||||||||
2 |
(S,S)-M3TAC |
ž |
151 |
|
|
|
|
|
|
|
|
|
|
|
ž |
223 |
|
ž |
236 |
ž |
||
|
|
|
|
|
|
|
|
|
|
|||||||||||||
3 |
(R,R)-M4TAC |
ž |
131 |
|
ž |
192 |
|
|
|
|
|
|
|
|
|
|
|
|
ž |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
4 |
(R,R)-M5TAC |
ž |
107 |
|
ž |
167 |
|
|
|
|
|
|
|
|
|
|
|
|
ž |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
5 |
(R,R)-M6TAC |
ž |
99 |
|
ž |
144 |
|
|
|
|
|
|
|
|
|
|
|
|
ž |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
6 |
(R,R)-M7TAC |
ž |
93 |
|
ž |
128 |
|
ž |
131 |
|
|
|
|
|
|
|
|
ž |
||||
|
|
|
|
|
|
|
|
|
|
|||||||||||||
(b) Racemic 1-methyl-alkyl-terephthalidene-bis-aminocinnamate |
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
n |
Compound |
C |
|
Sc |
|
|
|
So |
|
SC |
|
SA |
|
I |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
2 |
rac-M3TAC |
ž |
160 |
|
|
|
|
|
|
|
|
|
|
|
ž |
226 |
|
ž |
243 |
ž |
||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
3 |
rac-M4TAC |
ž |
137 |
|
ž |
145 |
|
ž |
201 |
|
ž |
202 |
|
|
|
|
ž |
|||||
|
|
|
|
|
|
|
||||||||||||||||
4 |
rac-M5TAC |
ž |
88 |
|
|
|
|
|
|
|
ž |
182 |
|
|
|
|
|
|
|
|
ž |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
5 |
rac-M6TAC |
ž |
85 |
|
|
|
|
|
|
|
|
|
158 |
|
ž |
175 |
|
|
|
|
ž |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
6 |
rac-M7TAC |
ž |
101 |
|
|
|
|
|
|
|
ž |
|
|
|
|
|
|
|
|
ž |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
7 |
rac-M8TAC |
ž |
87 |
|
|
|
|
|
|
|
|
|
145 |
|
|
|
153 |
|
|
|
|
ž |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
8 |
rac M9TAC |
ž |
83 |
|
|
|
|
|
|
|
ž |
|
|
|
|
|
|
|
|
ž |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
9 |
rac-M10TAC |
ž |
98 |
|
|
|
|
|
|
|
|
|
|
|
ž |
135 |
|
|
|
|
ž |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
10 |
rac-M11TAC |
ž |
79 |
|
|
|
|
|
|
|
ž |
126 |
|
|
|
|
|
|
|
|
ž |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
442 |
T. Hanemann and W. Haase |
Like in the closely related ferroelectric liquid crystals, one or more chiral centres in the molecule are essential, and the mesomorphic properties of the pure enantiomer or diastereomer differ from those of the racemic mixture. From Figure 18 one can depict a typical representative with antiferroelectric properties; again an azomethine unit and two cinnamoyl moieties form the central core of the molecule44. Table 11 compares the mesomorphic properties of the pure diastereomers with the racemic mixtures, both with different aliphatic chain lengths. In most cases the phase-transition temperatures of the mixtures are slightly lower than those of the pure chiral compounds, and increasing chain length decreases the clearing points dramatically.
V. MODERN TOPICS
A. Liquid Crystal Polymers
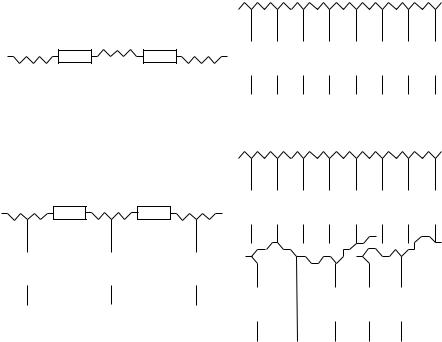
Hundreds of liquid crystalline polymers have been synthesized to date47,48. As compared with the low molecular mass LC’s, they have such advantages as easy processing, the capability to form fibres and films, and the possibility to have a liquid crystalline structure frozen in glass; but also such disadvantages as high viscosity and, therefore, much slower times of electro-optical response, which is important, e.g., for display applications. The four most typical structures of liquid crystal polymers (LCP) are shown in Figure 19. The chemical structure of the mesogenic moieties is in general the same,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) |
|
|
|
|
|
|
|
|
|
|
|
|
(b) |
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(c) |
(d) |
FIGURE 19. Main structural types of the LC polymers: (a) main chain, (b) side chain, (c) combination of (a) and (b), (d) crosslinked polymer networks
