
|
|
|
|
|
|
|
|
|
16. Heterogeneous catalytic hydrogenation |
|
883 |
|||||||||
TABLE 18. Characteristic data on the hydrogenation of cinnamaldehydea |
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
PhCH2 CH2 CHO |
(A) |
|
H2 |
|
|
||||
|
|
|
PhCH |
|
CHCHO |
H2 |
+ |
|
|
|
PhCH2 CH2 CH2 OH (31) |
|||||||||
|
|
|
|
(C) |
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
CHCH2 OH |
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
PhCH |
|
|
|
|
|
(B) |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
Catalyst |
Temp. |
Conversion |
|
|
Selectivity (%) |
Reference |
||||||||||
|
|
|
|
|
|
|
|
|
(K) |
(%) |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
A |
|
B |
C |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Co |
|
|
SiO2 |
303 |
|
50 |
|
|
|
|
|
93 |
384 |
|||||||
|
|
|
|
|
|
|
|
|||||||||||||
Ni2B |
298 |
|
100 |
99 |
|
|
|
|
385 |
|||||||||||
Ru |
|
|
KY |
373 |
|
25 |
16 |
17 |
|
67 |
379 |
|||||||||
|
|
|
|
|||||||||||||||||
Ru |
|
|
C |
333 |
|
40 |
30 |
10 |
|
60 |
386 |
|||||||||
|
|
|
|
|||||||||||||||||
Ru |
|
|
Sn |
|
C |
333 |
|
|
|
|
|
|
|
|
|
90 |
386 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
Rh |
|
|
KY |
373 |
|
25 |
47 |
20 |
|
33 |
379 |
|||||||||
|
|
|
|
|||||||||||||||||
Pt |
|
|
C |
333 |
|
70 |
80 |
5 |
|
10 |
387 |
|||||||||
|
|
|
|
|||||||||||||||||
Pt |
|
|
Nylon |
333 |
|
95 |
15 |
5 |
|
80 |
387 |
|||||||||
|
|
|
|
|||||||||||||||||
Pt |
|
|
Nylon C GeCl4 |
333 |
|
|
|
|
|
|
|
|
|
94 |
388 |
|||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Pt |
|
|
C C FeCl2 |
333 |
|
75 |
|
|
|
|
|
86 |
389 |
|||||||
|
|
|
|
|
|
|
|
|||||||||||||
a A: 3-phenylpropionaldehyde, B: 3-phenyl-1-propanol, C: cinnamyl alcohol (3-phenyl-2-propen-1-ol).
TABLE 19. Characteristic data on the hydrogenation of citral
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
O |
H2 |
|
|
|
|
|
H2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(A) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Z)-Citral |
|
|
Citronellal |
|
|
|
|
(E)-Citral |
|
|||
|
|
|
|
|
|
|
|
|
|
H2 |
|
|
|
H2 |
|
|
|
|
|
H2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
H2 |
|
|
OH |
|
|
H2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
(C) |
|
|
|
(B) |
|
|
|
|
|
(C) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
Nerol |
|
|
Citronellol |
|
|
|
|
Geraniol |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
SCHEME 12 |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Catalyst |
|
Temp. |
|
Conversion |
|
|
Selectivity (%) |
Reference |
||||||||||||
|
|
|
|
|
|
|
|
|
|
(K) |
|
(%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A |
|
B |
|
C |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Cu |
|
Cr |
|
|
|
|
oxide |
413 |
|
50 |
|
70 |
15 |
15 |
304 |
||||||
|
|
|
|
|
|
||||||||||||||||
Ru |
|
C |
|
|
|
333 |
|
90 |
|
30 |
35 |
35 |
386 |
||||||||
|
|
|
|
|
|
||||||||||||||||
Ru |
|
Al2O3 |
333 |
|
50 |
|
50 |
35 |
15 |
390 |
|||||||||||
|
|
|
|||||||||||||||||||
Ru |
|
Sn |
|
|
|
|
C |
333 |
|
30 |
|
15 |
5 |
80 |
386 |
||||||
|
|
|
|
|
|
||||||||||||||||
Rh |
|
SiO2 |
340 |
|
|
|
|
90 |
|
|
|
|
391 |
||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
Rh |
|
Ge |
|
|
|
SiO2 |
340 |
|
|
|
|
20 |
|
|
70 |
391 |
|||||
|
|
|
|
|
|
|
|
|
|
||||||||||||
Rh |
|
Sn |
|
|
|
SiO2 |
340 |
|
|
|
|
min. |
95 |
391 |
|||||||
|
|
|
|
|
|
|
|
||||||||||||||
884 |
´ |
Mihaly´ Bartok´ and Arpad´ Molnar´ |
the modification of catalysts with metal cations373,388,389,416,417,
the role of the bimetallic catalysts370,371,377,378,386,390,391,394,395,404,405,408,410,419,421, 422,424,425,
the effect of the modification of catalysts with sulfides and in other
ways369,371,374,384,407,416,
the effect of the supports369,375,378,379,384,394,396 400,408,410,415,417,425 including the role of SMSI372,376,378,384,396,406,408,409,415 ,
the role of the dispersion (particle size) of supported metal catalysts375,380,381,386,392 395,
413,414,417,420,423,
the role of the experimental parameters of the hydrogenation reaction (temperature, pressure, ratio of reactants, effect of solvents, etc.)369,374,376,388,400,402,403,409,411,416 ,
effect of the structure of the substrate369 371,373,374,379,385,413,417 ,
the mechanism of the hydrogenation reaction369,372,373,380 382,392,400,406,415,422 . These studies led to the following conclusions:
all metal catalysts which are active in the hydrogenation of alkenes and carbonyl compounds are also active in the hydrogenation of ˛,ˇ-unsaturated aldehydes,
due to thermodynamic reasons the reactivity of the unsubstituted CDC group in hydrogenation with unpromoted metals is generally higher than the reactivity of the aldehyde group,
saturated alcohols, formed in consecutive hydrogenation, are produced at high conversion,
the activity and the selectivity depend on the preparation conditions and the pretreatment of the catalyst, and the conditions of the hydrogenation (temperature, substrate H2 ratio, pressure, solvent, etc.),
metal particle size and morphology also affect selectivity,
the selectivity is strongly dependent on the nature of the metal and the crystal face, the adsorption through the CDO bond are preferred on metals which have a much larger
d-band width and a less filled d-band (Os, Ir),
the selectivity of the formation of ˛,ˇ-unsaturated alcohols can considerably be increased with various promoters (the highest selectivity could be achieved with nontransition elements),
the promoter can exert its activity only when it is on the metal surface; in the majority of cases, ionic species are responsible for the promoter effect,
the relative accessibility of the groups being hydrogenated and the binding strength to the catalyst of the CDC and CDO groups are important in determining selectivity,
the adsorption through the carbonyl group becomes more significant with increasing steric hindrance of the CDC group,
the trends in activity and selectivity of different catalysts indicate that the substituent effect are mainly steric in origin,
the enhancement in the selectivity of hydrogenation of unsaturated aldehydes on modified Cu catalysts is attributed to both geometric and electronic effects and the relative importance of these effects was found to depend on the nature of the organic substrate.
On the basis of the above conclusions, the selective hydrogenation of ˛,ˇ-unsaturated aldehydes to unsaturated alcohols can be achieved by taking into account the following considerations and factors:
modification of the metal catalysts with different additives to improve the adsorption and the reactivity of the CDO bond compared to those of the CDC bond,
promotion effects in the hydrogenation of unsaturated aldehydes depend on the way in which cations of the promoter activate the CDO bond and bind the CDC bond,
it appears that the key factor governing the selectivity of the formation of unsaturated alcohols is the tilting of the alkyl chain far from the surface,
16. Heterogeneous catalytic hydrogenation |
885 |
factors promoting the formation of ˛,ˇ-unsaturated alcohols:
the number and the steric demand of alkyl substituents (geometric effects), modification of supported metal catalysts with the addition of a second metal (bimetallic
catalysts) or ions (Lewis acids),
generation of SMSI on supported metals,
decrease in dispersion (increase in particle size) when supported metal catalysts are used,
when metal zeolite catalysts are applied, the increased basicity of the zeolite results in increased selectivity,
increase in the partial pressure of substrate.
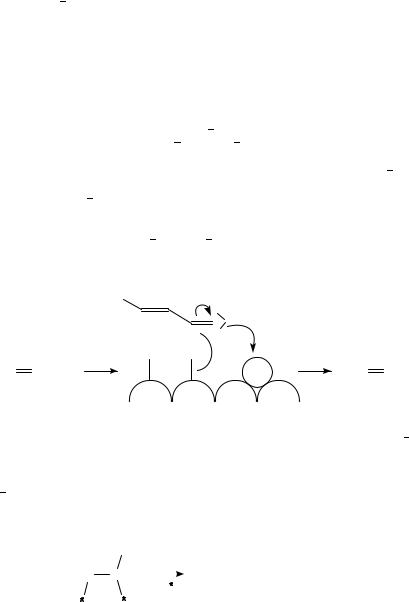
b. Mechanistic studies. It was shown378 that promotion of Pt by non-noble metals like Ni, Sn, Ga and Ti increased significantly the rate of hydrogenation of the carbonyl group of crotonaldehyde. The presence of Pt and of a metal with a fractional positive charge or a metal cation acting as electron pair acceptor site was shown to be indispensable for hydrogenation of the CDO group. Based on the decrease in the catalytic activity, it was concluded that two types of such a Pt promoter combination exist in the catalysts studied: the bimetallic phases of Pt Ni and Pt Sn, and the interface between Pt and nonstoichiometric titanium and gallium oxide particles. The enhancement of the rate of CDO group hydrogenation is believed to be caused by electron pair donor acceptor interactions between the positively charged sites and the carbonyl oxygen.
In the case of Pt Fe, the two metals were suggested389,421 to be in the metallic state forming an alloy. EXAFS studies indicated an electron transfer from Fe to Pt, which allowed the conclusion that the aldehyde double bond is more easily adsorbed on the ‘induced sites’. In the case of Pt Sn and Pt Ge, the authors suggested that SnnC or GenC ions (Lewis acids) activate the carbonyl group by enhancing the positive charge of the carbonyl carbon (equation 32).
R
δ+  O
O
H |
H |
|
(32) |
|
|
||
H2 |
|
δ+ |
RCH CHCH2 OH |
RCH CHCHO |
|
M |
|
Pt |
Pt |
δ− |
δ− |
Pt |
Pt |
The use of TiO2 as support significantly increases the rate of hydrogenation of the carbonyl group of crotonaldehyde to crotyl alcohol as compared to SiO2408,415. Pt TiO2 exhibited the highest activity for this reaction. In comparison to silica-supported catalysts it was shown that mainly the rate of CDO bond hydrogenation is enhanced, while differences in the specific activities of CDC bond hydrogenation were significantly smaller. The Pt Ti interface was suggested to be active for CDO group hydrogenation. The polarity of the active site is suggested to be responsible for the activation of the carbonyl group (equations 33 and 34).
CHO
H2 C CH |
H2 |
MeCH2 CHO |
(33) |
−2 |
|
||
|
|
|
886 |
|
|
|
´ |
|
Mihaly´ Bartok´ and Arpad´ Molnar´ |
|
||||
CH |
CH |
|
Ti+ |
MeCH2 CHO |
|
|
|
(34) |
|||
H2 C |
|
O |
|
H2 , − |
|
|
|
|
|
||
|
|
|
|
CH2 |
CHCH2 OH |
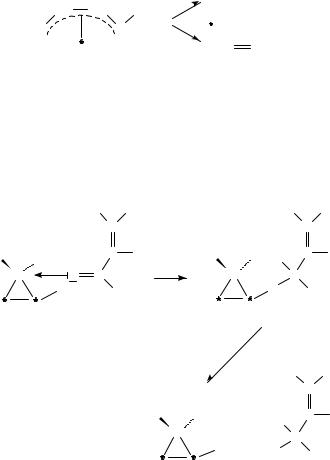
In the hydrogenation of cinnamaldehyde390, a higher selectivity to cinnamyl alcohol was observed on larger Ru particles. This was attributed to a steric effect of the aromatic ring which facilitates the adsorption of cinnamaldehyde through the CDO group on the larger metal particles. In the hydrogenation of citral, a compound without aromatic ring, such a steric effect does not exist. The effect of tin using modified catalysts is twofold390,421. One is to poison the Ru or Rh surface sites causing a decrease in the overall rate of reaction. The other effect is to activate the CDO group by facilitating the hydrogen transfer from adjacent Ru or Rh sites. The activating effect can be ascribed to the presence of tin ions which polarize the carbonyl group (equation 35).
|
|
|
H |
R |
|
|
|
|
H |
R |
|
|
|
C |
|
|
|
|
|
C |
|
Bu |
δ+ Bu |
δ− |
C |
H |
Bu |
δ+ |
Bu |
H |
C |
H |
|
C δ+ |
|
|
|
|
|
|
|||
|
Sn |
O |
|
|
Sn |
|
O |
C |
|
|
|
|
Hδ− |
H |
|
|
|
|
H |
|
(35)
|
|
|
|
H2 |
|
H |
R |
|
|
|
|
|
|
||
|
|
|
|
|
|
C |
|
Bu |
δ+ |
Bu |
|
+ |
H |
C |
H |
|
|
|
|
|
|||
|
Sn |
|
|
|
C |
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
HO |
H |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
The pore structure of the zeolites379 led to higher selectivity toward the unsaturated alcohol in the case of cinnamaldehyde, where steric constraints of the reactant prevented adsorption at the CDC bond on metal sites within the pores. 3-Methylcrotonaldehyde, in contrast, underwent less selective hydrogenation, since the mobility and orientation of this molecule was not inhibited by the zeolite pores. The selectivity of the formation of the unsaturated alcohol could be increased, however, by replacing NaC compensating cations in the zeolite with KC cations. This modification brought about a decrease in CDC hydrogenation attributed to enhanced metal electron density combined with an increase in CDO hydrogenation due to an interaction between the carbonyl function and the more basic zeolite cation.
The various experimental results with respect to the hydrogenation mechanism of ˛,ˇ- unsaturated aldehydes led to the proposal of the mechanism depicted in Scheme 13 with acrolein as a model compound372,427.
The nature of surface species, and consequently the reaction mechanism and thereby the selectivity, are determined by the substrate, the nature of the metal, the type of exposed crystal face and promoters as basic factors. The 2(C C), 2(C O) and 4(C C C O)
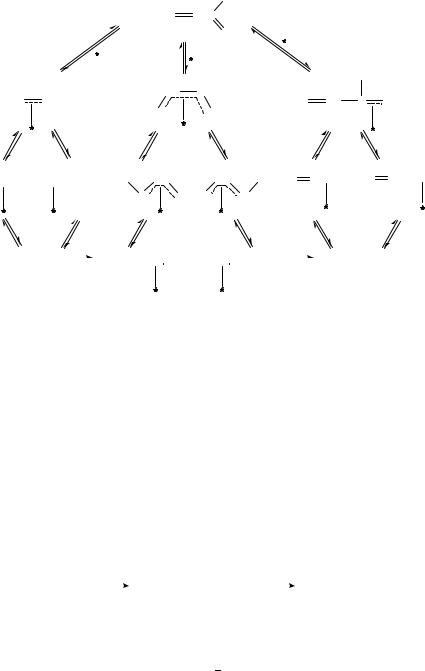
|
16. Heterogeneous catalytic hydrogenation |
887 |
|||||
|
|
|
|
H |
|
|
|
|
|
|
CH2 |
CHC |
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
H |
|
CH2 |
CHCHO |
|
CH |
CH |
CH2 |
CH C |
O |
|
CH2 |
O |
|||||
|
|
|
|
|
|
||
|
H |
|
H |
H |
|
H |
H |
H |
|
|
|
|
|
||
|
|
|
|
|
|
||
MeCHCHO |
CH2 CH2 CHO Me |
|
CH |
CH |
OH CH2 |
CHCHOH CH2 |
CHCH2 O |
|
|
CH |
O |
CH2 |
CH |
|
|
H |
|
H |
|
|
H |
H |
H |
|
H |
|
|
||||
|
|
|
|
|
|||
MeCH2 CHO  MeCH2 CH
MeCH2 CH

 O CH2
O CH2  CHCH2 OH
CHCH2 OH  CH2
CH2  CHCH2 OH
CHCH2 OH
SCHEME 13
surface species were also detected by different spectroscopic methods (See, e.g., References 420, 423).
2. Hydrogenation of unsaturated ketones
The number of papers on the hydrogenation of unsaturated ketones is significantly fewer than those on ˛,ˇ-unsaturated aldehydes. A possible reason is that the secondary alcohols formed by the hydrogenation of ketones are less important than the primary alcohols. In addition, the preparation of unsaturated secondary alcohols proved to be less successful although their significance as synthons in preparative organic chemistry is indisputable. The primary products of the catalytic hydrogenation of unsaturated ketones were shown to be the corresponding saturated ketones. Though these are important compounds their preparation by other well-known procedures is more economical.
˛,ˇ-Unsaturated compounds, the most extensively studied unsaturated ketones, are easily hydrogenated in both the liquid and the gas phase, even under mild experimental conditions (equation 36),
|
|
R2 |
R3 |
|
|
|
R2 |
|
R3 |
|
|
|
R2 |
|
R3 |
|||||||||||||||||||||||||
R1 |
|
|
|
|
|
|
|
|
|
|
|
|
H2 |
R1 |
|
|
|
|
|
|
|
|
|
|
|
|
H2 |
R1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
C |
|
C |
|
R |
|
CH |
|
CH |
|
C |
|
R |
|
CH |
|
CH |
|
CH |
|
R |
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH(36) |
||
|
|
|
|
|
O |
|
|
|
|
|
O |
|
|
|
|
|
|
|||||||||||||||||||||||
The catalysts used are Ni, Ru, Rh, Pd, Ir, Pt and Cu. The characteristic experimental results disclosed in recent publications are summarized in Tables 20 and 21. The majority of hydrogenations were carried out in the presence of Rh, Pt and Cu catalysts. Characteristic examples can be seen in equations 37 41, which also illustrate some exceptional behaviors.
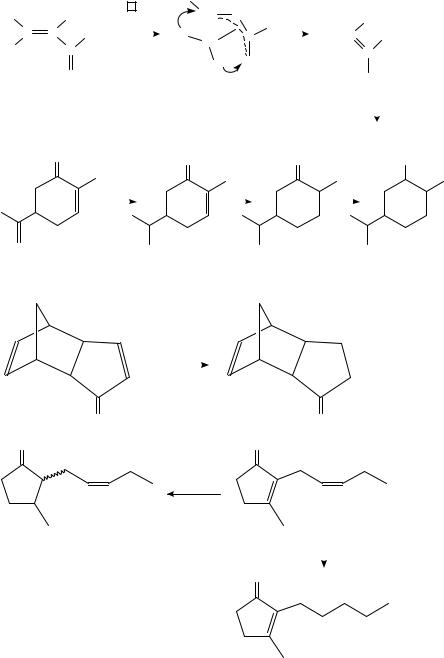
888 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
´ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mihaly´ Bartok´ and Arpad´ Molnar´ |
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ph |
|
|
|
|
|
|
|
≠ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ph |
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
CH |
CH |
R |
|
|
|
|
|
H |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
H |
|
Rh |
|
|
H |
|
|
|
|
|
|
|
|
|||||||||||||
C |
C |
R |
|
|
|
|
Rh |
C |
|
PhCH2 C |
|
|
R |
|||||||||||||||
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
−Rh |
|
|
||||||||||||||||
H |
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
O |
|
|
|
|
|
|
|
(37)432 |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PhCH2 CH2 COR |
|||
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
O |
|
|
|
|
|
OH |
|
|
|
Pt − SiO2 |
|
|
|
|
|
|
|
|
|
H2 |
|
|
|
|
|
|
H2 |
|
|
|
|||||
|
|
|
|
H2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(D) |
|
|
|
|
|
|
(A) |
|
|
|
(B) |
||
Carvone |
|
|
|
|
|
|
|
Carvotanacetone |
|
|
Carvomenthone |
|
Carvomenthol |
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(38)431 |
|
|
|
|
|
|
|
|
|
|
|
Cu − A l2 O3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
(39)56 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
H2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
||||||
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|||||
Cu − A l2 O3
H2
H2 |
Ni2 B |
(40)56,385 |
|
|
|
O
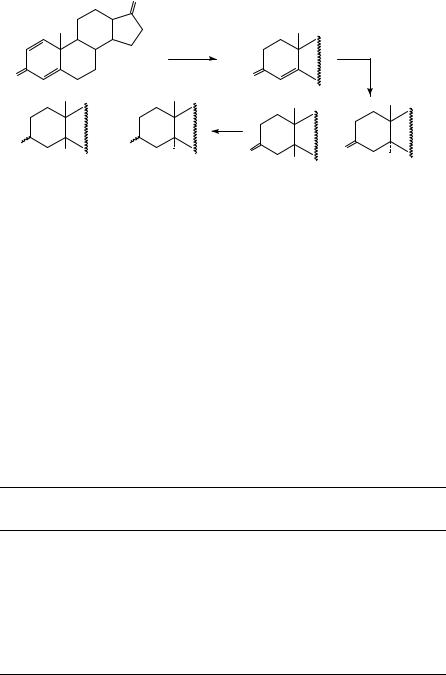
|
16. Heterogeneous catalytic hydrogenation |
|
|
889 |
|||
|
O |
|
|
|
|
|
|
|
Cu − A l2 O3 |
|
|
|
|
|
|
O |
H2 |
O |
|
|
|
H2 |
|
|
|
|
|
|
|||
+ |
|
H2 |
|
+ |
|
|
|
HO |
HO |
|
|
O |
|
|
|
O |
|
|
|
|
|||
H |
H |
β |
H |
α |
H |
||
|
|
|
|
||||
major |
(41) |
|
The investigation of hydrogenation reactions included the study of the following vari-
ables and problems: |
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
preparation and pretreatment of catalysts304,369,373,385,431,433 |
|
435, |
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
use of bimetallic catalysts373,428,431, |
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
the role of the support effect429,430,434, including SMSI206, |
|
|
|
|
||||||||||||||||
|
|
|
dispersion of the supported metal catalysts431,434, |
|
|
|
|
|
|
|
|||||||||||||
TABLE 20. Characteristic data on the hydrogenation of unsaturated ketonesa |
|
|
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
Catalyst |
Substrate |
Temp. |
Conversion |
|
|
Selectivity (%) |
|
Reference |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(K) |
(%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A |
B |
C |
D |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Cu |
|
|
Al2O3 |
Mesityl oxide |
323 |
100 |
8 |
88 |
|
4 |
369 |
||||||||||||
|
|
|
|||||||||||||||||||||
Cu |
|
|
Al2O3 C thiophene |
Mesityl oxide |
323 |
48 |
36 |
12 |
3 |
10 |
369 |
||||||||||||
|
|
||||||||||||||||||||||
Rh |
|
|
SiO2 |
Methyl vinyl ketone |
298 |
100 |
82 |
16 |
|
|
428 |
||||||||||||
|
|
|
|
||||||||||||||||||||
Rh |
|
|
Sn |
|
|
|
SiO2 |
Methyl vinyl ketone |
298 |
100 |
98 |
2 |
|
|
428 |
||||||||
|
|
|
|
|
|
|
|||||||||||||||||
Ir |
|
|
TiO2 (LTR) |
5-Hexen-2-one |
323 |
100 |
20 |
80 |
|
|
376 |
||||||||||||
|
|
|
|
||||||||||||||||||||
Ir |
|
|
TiO2 (HTR) |
5-Hexen-2-one |
323 |
17 |
82 |
6 |
|
12 |
376 |
||||||||||||
|
|
|
|||||||||||||||||||||
Pt |
|
Al2O3 |
Methyl vinyl ketone |
298 |
50 |
80 |
20 |
|
|
428 |
|||||||||||||
|
|
|
|||||||||||||||||||||
Pt |
|
Zr |
|
|
|
|
|
Al2O3 |
Methyl vinyl ketone |
298 |
20 |
100 |
|
|
|
428 |
|||||||
|
|
|
|
|
|
|
|||||||||||||||||
Pt |
|
|
SiO2 |
Methyl vinyl ketone |
353 |
low |
97 |
3 |
min. |
|
373 |
||||||||||||
|
|
|
|||||||||||||||||||||
Pt |
|
|
Fe |
|
|
|
|
SiO2 |
Methyl vinyl ketone |
353 |
low |
84 |
16 |
min. |
|
373 |
|||||||
|
|
|
|
|
|
||||||||||||||||||
Pt |
|
|
Sn |
|
|
|
SiO2 |
Methyl vinyl ketone |
353 |
low |
96 |
4 |
min. |
|
373 |
||||||||
|
|
|
|
|
|
||||||||||||||||||
a A: saturated ketone, B: saturated alcohol, C: unsaturated alcohol, D: other products.
TABLE 21. Characteristic data on the hydrogenation of cycloalkenonesa
|
|
|
|
|
|
|
|
Catalyst |
Substrate |
Temp. |
Conversion |
Selectivity (%) |
Reference |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
(K) |
(%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A |
B |
C |
D |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ni |
|
|
|
|
|
|
|
|
Verbenone |
373 |
100 |
100 |
|
|
|
429 |
||
Ni |
|
|
|
B |
Verbenone |
398 |
100 |
5 |
95 |
|
|
429 |
||||||
|
|
|
|
|
||||||||||||||
Ni2B |
Carvone |
298 |
100 |
>99 |
|
|
|
385 |
||||||||||
Cu |
|
|
|
Cr |
|
|
oxide |
2-Cyclohexen-1-one |
413 |
50 |
100 |
|
|
|
304 |
|||
|
|
|
|
|
|
|
|
|||||||||||
Cu on various supports |
˛-Ionone |
363 |
>90 |
90 |
|
|
|
430 |
||||||||||
Cu |
|
|
Al2O3 |
2-Cyclohexen-1-one |
353 |
100 |
<1 |
99 |
<1 |
0 |
369 |
|||||||
|
|
|||||||||||||||||
Cu |
|
|
|
Al2O3 C thiophene |
2-Cyclohexen-1-one 353 |
13 |
44 |
20 |
17 |
19 |
369 |
|||||||
|
|
|
||||||||||||||||
Pt |
|
|
SiO2 |
Carvone |
373 |
|
>50 |
|
|
<50 |
431 |
|||||||
|
|
|
|
|
||||||||||||||
Pt |
|
|
Au |
|
SiO2 |
Carvone |
373 |
|
<50 |
|
|
>50 |
431 |
|||||
|
|
|
|
|
|
|||||||||||||
a A: saturated ketone, B: saturated alcohol, C: unsaturated alcohol, D: other products.
890 |
´ |
Mihaly´ Bartok´ and Arpad´ Molnar´ |
|
the hydrogenation |
conditions (method, temperature, pressure, solvent, additives, |
etc.)56,369,373,429,435 437,
the decisive role of the substrate56,369,385,432,438,
the stereochemistry of hydrogenation430,432,434,435,438,439, the mechanism of hydrogenation432,434.
The most essential conclusions of the investigations are summarized below. Note that all considerations discussed in connection with the hydrogenation of ˛,ˇ-unsaturated aldehydes are also relevant to the hydrogenation of unsaturated ketones:
the hydrogenation activity of the metals investigated changes in the order Pt ¾ Pd ¾ Rh × Ru > Ni > Cu436,
on all catalysts, with the exception of Pd, saturated ketones are first formed through the selective hydrogenation of the olefinic double bond, then further hydrogenated to secondary alcohols,
the role of geometric-stereochemical factors in determining the selectivity of hydrogenation is even greater than in the hydrogenation of unsaturated aldehydes, if the hydrogenation of the CDO group is hindered by two bulky groups,
the only example describing the formation of an ˛,ˇ-unsaturated alcohol was the hydrogenation of 2-cyclohexen-1-one (ca 17% selectivity) over a thiophene-modified Cu Al2O3 catalyst369,
in the transformation of ˛,ˇ-unsaturated carbonyl compounds containing an additional isolated olefinic bond (carvone, ˛- and ˇ-ionone etc.) generally only the conjugated olefinic bond is hydrogenated,
a cis-concerted mechanism was assumed for the hydrogenation of (E)-benzylidene ketones over Rh sepiolite catalysts432 (equation 37),
highly selective formation of the ˛,ˇ-unsaturated alcohol occurs on MgO through transfer hydrogenation440.
VI. HYDROGENATION OF C=N BONDS
The CDN bonds of imines, oximes and hydrazones can be hydrogenated to form the corresponding amines even under ambient conditions on Pt, Pd, Rh and Raney Ni catalysts in acidic, neutral or basic media (equation 42). The imines, furthermore, are intermediates in the hydrogenation of nitro compounds, nitriles and oximes, and likewise play a key role as intermediates in the reductive amination of carbonyl compounds.
R1 |
|
|
|
R1 |
|
C N X |
M, H2 |
CHNH2 |
|
|
|
|
||
R2 |
|
|
|
R2 |
|
|
|
|
(42) |
R1 = alkyl, cycloalkyl, aryl |
|
|||
R2 |
= H, alkyl, cycloalkyl, aryl |
|
||
X = H, OH, OR, OAc, NH2 , NHR |
|
|||
The general characteristics of the hydrogenation of compounds possessing CDN bonds were already described in the 1940s and the reaction has been applied in preparative organic chemistry. The results were summarized in monographs315,441 446. In recent years mainly the asymmetric hydrogenation of the CDN bond has been studied.
The use of an acidic medium, in general, is favorable for the hydrogenation with any of the above catalysts. This is presumably connected with the elimination of the inhibitory effect of the amines formed during the hydrogenation. In imine hydrogenation reactions on Rh, fiveto eightfold rate increases are observed when tartaric, phthalic, mandelic, salicylic or formic acids are added to the alcoholic reaction mixture (in a 95:5 mixture of EtOH MeOH)447.
16. Heterogeneous catalytic hydrogenation |
891 |
Aldimines are generally more easily hydrogenated than ketimines, due to the steric hindrance arising with the latter compounds444. The rate of hydrogenation of imines and the product composition are determined by the structure of the imine448.
The stereochemistry of the hydrogenation strongly depends on the catalyst and the reaction conditions. Some examples illustrating the stereochemistry of the hydrogenation of imines are given in equations 43 47.
|
R |
|
|
|
|
|
|
N |
|
|
NHR |
|
|
|
Pd, H2 |
|
|
|
|
(43)449 |
Me |
dioxane |
|
Me |
|
|
|
Me |
Me |
|
Me |
Me |
|
|
|
|
|
|
|
||
R = Me, p-MeOC6 H4 |
|
|
|
|
|
|
|
Pt, H2 |
|
|
H |
|
(44)450 |
|
EtOH |
|
|
|
|
|
|
NPh |
|
NHPh |
|
|
|
Me |
Me |
Me |
Me |
|
|
|
|
Pt, H2 |
|
|
|
|
(45)450 |
Me |
EtOH |
Me |
|
NHPh |
|
|
NPh |
|
|
|
|
||
|
|
H |
|
|
|
|
Me |
Me |
Me |
Me |
|
|
|
|
Pt, H2 |
|
|
|
|
(46)451 |
Me |
A cOH |
Me |
|
NH2 |
|
|
NH |
|
|
|
|
||
|
|
H |
|
|
|
|
|
Me |
|
|
Me |
|
|
|
n |
|
|
n |
|
|
|
|
Pd − C, H2 |
|
|
|
|
|
N |
MeOH |
|
H |
N |
(47)452,453 |
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
R |
|
|
R |
|
|
|
|
R = H, MeO |
|
|
trans/cis = 89−100/11−0 |
|
|
The high stereoselectivity results from the attack of hydrogen from the sterically less hindered side during cis addition.
The heat of hydrogenation of 1-azacyclopentene was determined by measuring the heat of hydrogenation of its trimer. The data give information on the heats of formation and strain energies of a number of cyclic and acyclic imines454.
892 |
´ |
Mihaly´ Bartok´ and Arpad´ Molnar´ |
There has been no new information on the hydrogenation of oximes in recent years. A detailed summary of this field was published in 1985446.
VII. ASYMMETRIC HYDROGENATIONS
The asymmetric hydrogenation of double-bonded functional groups has recently become of great practical importance.
Heterogeneous metal catalysts can be modified for chiral synthesis in two general ways. Either supported metals are treated with chiral modifiers or metal catalysts are prepared by using chiral supports. Two systems have been developed into highly asymmetric heterogeneous hydrogenation catalysts. Systematic studies on the chiral heterogeneous catalytic hydrogenation were carried out using Raney Ni modified with various chiral reagents. (2R,3R)-(C)-Tartaric acid was found to be the best chiral modifying reagent in the presence of NaBr co-modifier. The other system is the cinchona alkaloid modified Pt catalyst.
The heterogeneous catalytic hydrogenation of carbonyl compounds using chirally modified metal catalysts has been reviewed in recent years315,455 459. The conclusions can be summarized as follows:
not only Ni and Pt but several other metals (Co, Fe, Ru, Pd, Rh, Cu) have been investigated,
the substrates used were mainly ˛- and ˇ-keto esters, as well as 1,2- and 1,3-diketones, on the TA NaBr MRNi catalysts the ee values on hydrogenation of ˇ-keto esters are
88 92%,
the cinchona alkaloid modified Pt catalysts used for the chiral hydrogenation of ˛-keto esters giving ˛-hydroxy esters with ee values up to 95%,
the optical purity of the product is dependent not only on the preparation method of the catalyst (quantity and type of modifier, impregnation methods, support, catalyst dispersion, metal source etc.) but also on the reaction conditions (H2 pressure, solvent, reaction temperature, substrate concentration and others),
catalysts with the largest metal particle sizes gave the highest enantioselectivity,
the solvent has a large effect on the reaction (aprotic solvents like THF and various esters are the best),
additives (inorganic salts, water, organic acids) enhance the ee values,
despite the large amount of published data there is still no agreement on the nature of the chiral hydrogenation site and the origin of the observed enantio-differentiation.
The majority of recent publications still deal with the chiral hydrogenation of ketones containing other functional groups as well. The general characteristics of these reactions are illustrated by the asymmetric hydrogenation of ˛,ˇ-unsaturated ketones. In addition, the purpose of the present review is to summarize the latest results of the chiral hydrogenation of ketones which do not contain other functional groups (dialkyl ketones and alkyl aryl ketones).
The chiral hydrogenation of dialkyl ketones with high ee (80%) was performed on the TA NaBr MRNi catalyst in the presence of pivalic acid as co-modifier460 465. The first substrates examined were 2-alkanones (equation 48).
R |
|
C |
|
Me |
TA − NaBr− MRNi, H2 |
R |
|
CH |
|
Me |
|
|
|
|
pivalic acid,
O 323-333 K, OH (48) THF
R = Et, Bu, Pen, Hex, octyl, undecyl
The preparation of the catalyst and the effect on the ee of hydrogenation conditions (temperature, solvent, the ratio of substrate to solvent, concentration of co-modifier) were
