

Supplement A3: The Chemistry of Double-Bonded Functional Groups. Edited by Saul Patai Copyright 1997 John Wiley & Sons, Ltd.
ISBN: 0-471-95956-1
CHAPTER 18
Nucleophilic attack on compounds containing C=C, C=O or C=N groups
PETER G. TAYLOR
Chemistry Department, Open University, Walton Hall, Milton Keynes, Bucks, MK7 6AA, UK
Fax: 01908 653744; e-mail: P.G.Taylor@Open.ac.uk
I. INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1104 |
II.THE TYPES OF DOUBLE BONDS INVOLVING AT LEAST ONE CARBON ATOM THAT UNDERGO NUCLEOPHILIC ATTACK AND
THEIR RELATIVE REACTIVITIES . . . . . . . . . . . . . . . . . . . . . . . |
1104 |
|
A. Nucleophilic Attack on Carboxylic Acid Derivatives . . . . . . . . . . . . |
1105 |
|
B. Nucleophilic Attack on Alkenes . . . . . . . . . . . . . . . . . . . . . . . . . |
1106 |
|
C. Conjugate Additions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1107 |
|
1. |
Mechanistic considerations . . . . . . . . . . . . . . . . . . . . . . . . . . |
1107 |
2. |
Relative reactivities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1108 |
D. Nucleophilic Attack on Allyl Systems. . . . . . . . . . . . . . . . . . . . . . |
1110 |
|
E. Nucleophilic Attack on Halogenated Alkenes . . . . . . . . . . . . . . . . |
1111 |
|
F. Nucleophilic Attack at Other Heterosubstituted Alkenes . . . . . . . . . |
1112 |
|
III. THE ORBITAL INTERACTIONS THAT CONTROL THE APPROACH OF |
|
|
A NUCLEOPHILE TO THE DOUBLE BOND . . . . . . . . . . . . . . . . . |
1113 |
|
IV. STEREOSELECTIVITY IN NUCLEOPHILIC ATTACK OF DOUBLE |
1119 |
|
BONDS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
|
A. Diastereoselective Approach of the Nucleophile on the |
|
|
Double Bond . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1119 |
|
B. Stereoselectivity as a Result of a Chiral Centre ˛ to a Carbon Double |
|
|
Bond in the Substrate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1119 |
|
C.Stereoselectivity of Nucleophilic Attack on Cyclic Ketones. . . . . . . . 1124
D.Diastereoselectivity as a Result of a Chiral Centre ˇ to a Carbon Double
Bond in the Substrate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1127 |
E. Stereoselectivity of Conjugate Additions . . . . . . . . . . . . . . . . . . . |
1128 |
V. REFERENCES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1131 |
1103
1104 |
Peter G. Taylor |
I. INTRODUCTION
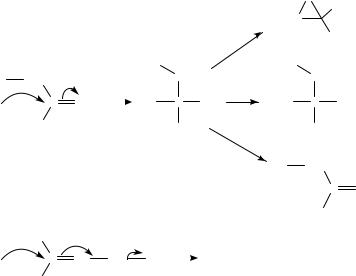
The topic of nucleophilic attack at an sp2 carbon would be too wide a field to review in one chapter, if one were to discuss all facets and outcomes, some of which are shown in Figure 1. If the nucleophile is anionic an anion is formed, but if the nucleophile is neutral a zwitterion is produced. If X is oxygen or nitrogen, then the outcome can be carbonyl addition, substitution or a Darzens-type reaction. If the X is another carbon then addition leads to conjugate additions or polymerization. Alternatively the nucleophile could be expelled after rotation about the C C bond to give overall isomerization. Both substitution and cyclization are also observed.
To make the task more manageable this chapter will focus specifically on the interaction between the nucleophile and a double bond and not consider in any depth subsequent steps. We will also only briefly consider reactions in which there is a preassociation or complexation of the double bond with a Lewis acid prior to nucleophilic attack. Finally we shall concentrate on ‘conventional’ nucleophilic attack and not discuss mechanisms involving single electron processes. In Section II we shall examine the types of double bonds that undergo nucleophilic attack, in particular examining relative reactivity, where available, and models for explaining this order. In Section III we shall review the orbital interactions that control the approach of a nucleophile to the double bond and the associated geometrical constraints. Then in Section IV we shall consider the implications of these constraints on selective reactions.
II. THE TYPES OF DOUBLE BONDS INVOLVING AT LEAST ONE CARBON
ATOM THAT UNDERGO NUCLEOPHILIC ATTACK AND
THEIR RELATIVE REACTIVITIES
The system of double bonds represents a region of high electron density such that, in the absence of other factors, double bonds are more prone to electrophilic attack. However,
|
|
|
|
|
X |
|
|
|
|
|
Y |
|
|
|
|
RCH |
|
|
|
|
|
|
Nu |
|
|
|
R |
R |
|
|
R CHZ |
CHZ |
|
CHZ |
|
|
|
|
|
||
Nu− |
C X |
|
Nu C X− |
Nu |
C XE |
|
|||||
|
|
|
|
|
|
|
Y |
Y |
|
Y |
|
|
|
|
|
||
|
X = O, N, CH−W |
|
R |
CHZ |
|
C X
|
|
|
|
|
|
|
|
|
|
Nu |
|
R |
|
|
|
R |
|||||
|
C CH CH2 X |
|
|
|
|
|
|
|
|
|
− |
|
Nu |
|
C |
|
CH |
|
CH2 |
||
|
|
|
|
|||||||
|
|
|
||||||||
|
|
|
|
|||||||
Nu |
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
||||||
|
|
|
|
|
||||||
FIGURE 1. Nucleophilic attack of CDX and CDC and possible outcomes

18. Nucleophilic attack on compounds containing CDC, CDO or CDN groups 1105
Aldehydes and ketones |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
> |
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
C |
|
|
H |
|
|
|
|
|
|
R |
|
|
|
|
|
C |
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
Carboxylic acid derivatives |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
O |
|
|
|
|
O |
|||||||||||||||||||||||||||||||||
|
|
|
|
+ |
> |
|
|
|
|
|
|
|
|
|
|
|
> |
|
|
|
|
|
|
|
> |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
> |
|||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
C |
|
Cl |
|
|
|
|
|
|
|
R |
|
|
C |
|
SR |
R |
|
C |
|
O |
|
C |
|
R |
||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||
R C NR3 |
|
|
|
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
(1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2) |
|
|
|
|
|
|
|
|
|
|
(3) |
|
|
|
|
|
|
|
|
|
|
|
|
|
(4) |
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|||||||||||||||||||||
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
> |
|
|
|
|
|
|
|
|
|
|
|
|
|
> |
|
|
|
|
|
|
|
|
|
|
|
|
|
> R |
|
|
|
|
|
|
|
|
|
|
O− |
||||||||||
C |
|
|
|
|
|
OR |
|
|
|
|
R |
|
|
|
C |
|
|
|
|
|
NHAr R |
|
C |
|
|
|
NHR |
C |
|
|
|||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
(5) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(6) |
|
|
|
(7) |
|
|
|
|
|
|
|
(8) |
|
|
|||||||||||||||||||||||||||||||
Carbon−carbon double bonds
H2 C CHX
CHX
where X is:
NO2 > PhCO > SO3 Ph > CHO > MeCO > CO2 Ph > p-MeC6 H4 SO2 > CN > SO2 NMe > CO2 Me > SOPh > 4-pyridyl > PO(OCH2 CH2 Cl)2 > CONH2 > PO(OEt2 ) > CONHR> p-NO2 C6 H4 > SPh > Cl > H
FIGURE 2. Examples of carbon double bonds that are prone to attack by nucleophiles and their relative reactivities
when the electron distribution is polarized in some way such that one carbon of the double bond becomes more electrophilic, then attack by a nucleophile will be possible. Obvious cases of this are CDX species such as aldehydes, ketones, carboxylic acid derivatives and imines. However, carbon carbon double bonds are also prone to nucleophilic attack when one of the carbons is attached to groups which can polarize the electron distribution of the double bond. In terms of a ground state argument, nucleophiles will attack a CDX to give Nu C X , if negative charge formation at X is favourable. Again obvious cases are when X is oxygen or nitrogen or suitably substituted carbon, such as a carbon adjacent to a carbonyl where X represents an enolate. Of course such arguments involve many simplifications and the range of carbon double bonds attacked by nucleophiles will depend upon such things as the nucleophilicity of the attacking species, solvents, proximity etc. Similarly in some nucleophilic attacks at carbon double bonds Nu C X species will not appear as intermediates, for example concerted substitutions at sp2 carbons.
Figure 2 shows the range of carbon double bonds that are prone to nucleophilic attack by conventional nucleophiles together with a measure of relative reactivity where available.
A. Nucleophilic Attack on Carboxylic Acid Derivatives
Theoretical calculations using MNDO and 4-31G basis sets of the frontier orbitals by the group of Yamabe1 have shown that nucleophilic attack of carboxylic acid derivatives
1106 |
Peter G. Taylor |
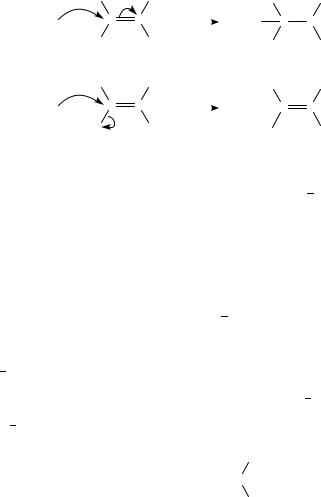
in the gas phase can proceed via a tetrahedral intermediate or in a concerted process with the carbon-leaving group bond breaking as the carbon nucleophile bond is made (Scheme 1).
|
R |
|
|
R |
|
R |
|
|
|
|
|
|
|
||
|
|
|
|
− |
−X− |
||
Nu− |
C O |
|
Nu |
C O |
|
|
C O |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
X |
|
|
X |
|
Nu |
|
|
|
|
|
|
|
||
|
|
R |
|
R |
|
|
|
|
|
C |
O |
−X− |
O |
||
|
Nu− |
C |
|||||
|
|
|
|
|
|
|
|
|
|
X |
|
Nu |
|
|
|
 Y
Y
 X−
X−
O
C Z
φ
CH3
Cl
SCHEME 1
Nucleophilic attack involves electron donation to the Ł orbital of the carbonyl; however, the relative reactivity does not reflect the order of these LUMO energy levels. To account for the order it is necessary to consider both the bond-making and bondbreaking processes, that is we need to mix the LUMO with the LUMO C 1 ( CŁ X). The level of mixing depends upon how close the two LUMOs are. Calculations show that the correct order of energy levels is obtained when ϕ is 20°. The difference in energy between the LUMO and the LUMO C 1 also predicts whether there is an intermediate along the pathway or not. When the energy difference is small, usually associated with a weak C L bond, no intermediate is predicted (1 4); however, when the energy difference is large an intermediate is predicted (5 8). Contrary to this conclusion, pulsed ion cyclotron resonance spectroscopy studies of nucleophilic displacements in the gas phase suggest that acyl halides undergo substitution via a tetrahedral intermediate with a range of nucleophiles2.
B. Nucleophilic Attack on Alkenes
Whilst nucleophiles do not generally add to unactivated double bonds, they do react if the two groups are close together as part of the same molecule. Evans and Kirby have examined the intramolecular nucleophilic addition of phenolate to unactivated double and triple bonds3,4. The nucleophilic phenolate oxygen adds to the double bond up to the point where bond formation, and hence the formation of the primary carbanion, is well advanced. The intermediate ion, 9, would normally revert to starting material but in the presence of a general acid it can be protonated to give a stable product. Thus, the general acid plays an essential role in avoiding the formation of a complete primary alkyl carbanion in water.
18. Nucleophilic attack on compounds containing CDC, CDO or CDN groups |
1107 |
||
|
CH2 |
− |
CH3 |
O− |
O |
O |
|
HA
H2 O
(9)
As expected from Baldwin’s rules, with suitable substrates, both regiospecific 5- or 6-exo addition could be observed. The electronically symmetrical alkene 10 underwent both 5-exo and 6-endo-trig addition in a ratio of 18:1. This is in accord with Baldwin’s rule in that, whilst both processes are favourable, five-membered rings are formed more readily than six-membered rings. They also showed that 6-endo-trig and 6-exo-trig were both favourable processes with rate constants differing by a factor of less than 3.
HO
Br
(10)
Similar reactions have been reported in bicyclic systems where the intramolecular reaction of an alcohol with a simple alkene leads to relief of ground state strain5.
C. Conjugate Additions
1. Mechanistic considerations
Rappoport has carried out extensive work on nucleophilic substitution reactions. Several excellent reviews have appeared so only brief details are given here6,7. There are two possible mechanisms that involve initial attack of the nucleophile, which mirror the behaviour of acyl chlorides: initial attack of the nucleophile to give a carbanion followed by loss of the leaving group or a concerted attack of the nucleophile with loss of the nucleophile (Scheme 2). The former predominates in systems where Y1 and Y2 are good electron withdrawers and the leaving group is poor. The latter predominates with a good leaving group and poor electron-withdrawing characteristics of Y1 and Y2. The electron-withdrawing characteristics of Y1 and Y2 can be evaluated from the pKa of the carbon acid CH2Y1Y2. Based on a variety of evidence, such as the stereochemistry of the substitution and kBr/kCl and kCl/kF element effects, Rappoport proposed that for poor leaving groups, the vinyl substitution would be multistep if the pKa of the corresponding carbon acid CH2Y1Y2 were less than 40. With good leaving groups, such as Cl, Br and
1108 |
|
|
Peter G. Taylor |
|
|
|||
|
R |
|
Y1 |
R |
Y1 |
|||
Nu− |
C |
C |
|
|
Nu |
C |
C − |
|
|
|
|||||||
|
X |
|
Y2 |
X |
Y2 |
|||
|
R |
|
Y1 |
R |
Y1 |
|||
Nu− |
C |
C |
|
|
|
C |
C |
|
Y2 |
||||||||
|
X |
|
Nu |
Y2 |
||||
SCHEME 2
OTs, the vinyl substitution would be multistep if the pKa of the corresponding carbon acid CH2Y1Y2 were less than 20. If the pKa were in the range 20 30 he suggests that the evidence points to a multistep process. However, if the pKa were greater than 40 the substitution would probably be a single step.
In a third review Rappoport points out the difficulty of trying to construct even a qualitative nucleophilicity scale towards a vinylic carbon8. Whilst for a range of nucleophiles, plots of log k versus a parameter which reflects the nucleophilicity, such as NC , are sometimes parallel for a range of related alkene substrates, for the majority of reactions studied a constant selectivity relationship does not apply. Each substrate requires a different blend of steric, polar and hard/soft nucleophile arguments to describe the relative reactivities. Bernasconi has correlated the reactivity of carbon carbon double bonds of the type 11 with the intrinsic rate constant for deprotonation of 129. Plots of the logarithm of one rate constant versus the logarithm of the other rate constant give reasonably straight lines with a slope which reflects the smaller change in the rate constant for nucleophilic attack on the carbon carbon double bond. He suggests that in both these reactions the development of resonance and the concomitant solvation of the carbanion lags behind other bond changes and that this lag is less extreme for nucleophilic attack of a carbon carbon double bond than for proton transfer. He suggests that this lag can be used to explain much of the structure reactivity behaviour of such systems.
X
RCH  CXY H2 C
CXY H2 C
Y
(11)(12)
where X and Y are H, CN, COCH3 , NO2 , PhNO2
2. Relative reactivities
Shenhav, Rappoport and Patai examined the kinetics of addition of morpholine and pyrrolidine to various activated olefins in methanol and obtained an order similar to Figure 1, as shown in Table 110. They pointed out that data in the literature suggested that CO2Me and CN are sometimes reversed11.
Heo and Bunting have examined the rate-determining steps in conjugate additions and E1cb reactions in aqueous solutions12. They examined the reactions between a range
18. Nucleophilic attack on compounds containing CDC, CDO or CDN groups 1109
TABLE 1. The relative rates for nucleophilic addition to electrophilic alkenes
Alkene |
k (rel)a |
k2 (rel)b |
k (rel)c |
||
CH2DCHCOPh |
11300 |
|
|
|
|
CH2DCHSO3Ph |
9000 |
|
|
|
|
CH2DCHCHO |
3600 |
3900 |
|
|
|
|
|
|
|||
CH2DCHCOCH3 |
3400 |
900 |
630 |
||
CH2DCHSO2CH3 |
|
140 |
50 |
||
CH2DCHCO2Ph |
400 |
|
|
|
|
CH2DCHCO2CH3 |
29 |
25 |
29 |
||
CH2CHCN |
16 |
6 |
8 |
||
CH2DCHCONH2 |
1 |
1 |
1 |
||
a Relative rate constants for the addition of morpholine to CH2DCHZ in methanol10.
b Relative rate constants for the addition of 4-(dimethylamino)pyridine to CH2DCHZ in aqueous solution12.
c Relative rate constants for the addition of glycine to CH2DCHZ in aqueous solution13.
of conjugated double bonds and substituted pyridines and were able to obtain rate and equilibrium data for each of the steps. The relative rates of nucleophilic attack are also given in Table 1. They found that the rates of nucleophilic attack upon these alkenes are much more sensitive to the nature of Z than are the equilibrium constants for addition. The data presented agreed with previous data of Rappoport and Patai10 and also with data for the addition of glycine to CH2DCHZ in aqueous solution13.
N + |
CH2 |
|
CHZ |
k |
2 |
|
|
|
|||
|
k−2 |
||||
X |
|
|
|
||
|
|
|
|
|
|
+ −
NCH2 CHZ
X
k−1 k1 [−OH]
+
NCH2 CH2 Z
X
By examining the rates of intramolecular nucleophilic cyclization of 13 at different pH values, Kirby has determined the relative reactivity of RCHDCH2, RCHDCHCOO and RCHDCHCOOH towards nucleophilic substitution14. The ratios 1:4000:8 ð 107 are in broad agreement with the relative rates, 3 ð 104:1, for nucleophilic attack of ammonia on fumaric acid in the neutral and dianionic forms at 135 °C. The relative rates for nucleophilic attack of hydroxide on fumaric acid in the neutral and dianionic forms at 135 °C changed to 3 ð 107:1 because of the adverse electrostatic interaction between the hydroxide ion and the dianion15.
Calculations of reactions of simple nucleophiles such as H with carbonyl compounds show that, in the gas phase, there is no barrier. Activation energies in solution arise from desolvation of the nucleophile. However, reactions of a nucleophile with an alkene or an alkyne do have a barrier in the gas phase.
The group of Osman16 has reported that the incoming nucleophile will align its region of charge concentration with the largest region of charge depletion in the valence shell
1110 |
Peter G. Taylor |
CO2 H
OH
X
(13)
of the electrophile. For acrylic acid systems the nucleophile attacks C(1) above or below the plane at an angle of 115°. Relative reactivities of acrylates to nucleophilic attack are related to the regions of charge depletion on C(1) as given by the minimum in r2p in the valence shell of charge concentration at C(1). This gives the order acrolein, acrylic acid, acrylonitrile and methacrylic acid. The net charge on the electrophilic centre q(C(1)) is not a good parameter of reactivity of C(1) to nucleophilic attack.
For attack of F on acrylic acid, first a hydrogen bonded complex is formed which then proceeds to the transition state and then to a stable carbanion. The methyl in the methacrylic acid reduces stabilization of the carbanion as predicted. Subsequent studies using ammonia as the nucleophile indicated that attack proceeded by a rate-determining intramolecular proton transfer from the nucleophile to the ligand, assisted by a discrete water molecule that acts as a catalyst17. They predicted that acrolein underwent 1,4- addition, acrylic acid either 1,2- or 1,4-addition and acrylonitrile 1,2-addition.
The relative reactivities of acrolein, acrylonitrile, methyl acrylate and methyl methacrylate have also been investigated by means of the Fukui function fC (r) and its condensed
counterpart fcC18 . These are local properties that help determine the preferred direction for a reagent to approach a substrate. In this instance they also mirror the relative reactivity of different substrates. Both functions correlated well with the experimental data, the LUMO density being a relatively good approximation to the Fukui function. A closely related local property is the condensed local softness sC (r), which also correlated well with the relative reactivities19.
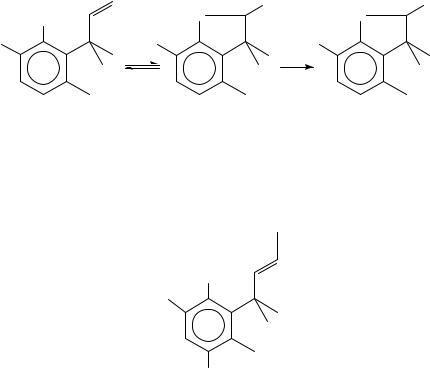
D. Nucleophilic Attack on Allyl Systems
Nucleophilic attack at the double bond of an allylic system bearing an ˛-leaving group has received special attention and has been reviewed extensively20,21, so only brief details will be given here. Various pathways for substitution have been proposed; however, we shall only concern ourselves with attack at the carbon of the double bond with formation of the carbanion or concerted loss of the leaving group. Magid20 has thoroughly reviewed the evidence for the mechanism: suffice it to say that some authors favour a concerted process22,23 whilst others think it a ‘myth’ and favour the formation of discrete intermediates24. There is also competing attack at the carbon ˛ to the leaving group, the extent of which depends upon the presence and size of substituents at the ˛ and positions. Syn stereochemistry is usually observed with the nucleophile attacking from the same side that the leaving group departs, but the results are often complicated by conformational bias25. The preference for syn attack is supported by calculations20. Nevertheless, anti
18. Nucleophilic attack on compounds containing CDC, CDO or CDN groups 1111
behaviour has been observed26. Calculations also suggest that soft nucleophiles give syn stereochemistry27 but anionic nucleophiles give anti stereochemistry28.
Intramolecular nucleophilic attack at the double bond of an allylic system bearing an ˛-leaving group has been extensively reviewed by Paquette and Stirling21. One of the key aspects of such reactions is the limitation of ring size on the trajectory of the nucleophile on the carbon double bond, although examples of 3,4,5 and 6 exo-trig reactions are known (for example see Scheme 3)29. The stereochemistry of these intramolecular reactions mirrors that of the intermolecular reactions in that the attack is usually, but not always, syn.
(EtOOC)2 CH |
|
(EtOOC)2 C− |
Br |
NaOBu-t |
|
|
Br |
|
|
ether |
|
CH3 |
|
|
|
CH3 |
|
|
|
COOEt
COOEt
CH3
SCHEME 3
Vinylsulphonyl chlorides undergo a similar reaction. The sulphonyl group activates the double bond to nucleophilic attack with loss of a chlorine to give a sulphene30. It was not possible to distinguish whether a two-step process via a zwitterion or a concerted process was involved.
X
N
CH2  CHSO2 Cl
CHSO2 Cl
X
+
NCH2 CH SO2
E. Nucleophilic Attack on Halogenated Alkenes
Another series of carbon carbon double bonds that are prone to nucleophilic attack are halogenated alkenes, where the developing carbanion is stabilized by inductive withdrawal by the halogen. Alkenes bearing fluorines are more prone to nucleophilic attack than the corresponding chloro derivatives. In perfluoro compounds nucleophilic attack occurs preferentially at a terminal CF2D group and the presence of perfluoroalkyl groups on the double-bond carbon ˇ to attack increases the reactivity due to the formation of a more stable carbanion31. This can also be explained using a Frontier Orbital approach, where the presence of the trifluoromethyl group lowers the energy of the LUMO32. The

1112 |
Peter G. Taylor |
corresponding perfluoro dienes also undergo nucleophilic attack. 14 undergoes nucleophilic attack (Scheme 4) much more quickly than 15 due to the relief of angle strain during formation of the intermediate carbanion33. Interestingly, both double bonds undergo nucleophilic attack with overall substitution. This has been used to form fluorinated heterocycles and cyclopentadienes34.
|
|
|
|
|
|
MeO |
F |
|
|
|
|
|
|
|
|
|
|
|
MeO |
|
F |
|
|
|
|
|
F |
|
|
|
|
|
||
(14) |
|
|
|
|
|
MeO |
|
|
|
|
|
|
|
F |
F |
|
|
|
F |
|
|
|
|||||
(15) |
|
|
|
|
|
|
CF3 |
|
|
|
|
|
|
F |
CF3 |
|
|
CsF |
||
|
+ |
PhNH2 |
||||
|
|
|||||
|
|
|||||
|
|
|
|
|
7 Days, R.T. |
|
CF3 |
F |
|
|
|
||
|
CF3 |
|
|
|
||
|
|
|
|
|
SCHEME 4 |
|
F
MeO |
|
F |
|
CF3 |
CF3 |
C |
C |
C |
C |
CF3 |
CF3 |
|
N |
|
Ph |
|
(72%) |
F. Nucleophilic Attack at Other Heterosubstituted Alkenes
Trimethylsilyl groups stabilize ˛ carbanions and thus promote nucleophilic attack at the ˇ position of a double bond. Stork has used ˛-silylsubstituted vinyl ketones extensively in conjugate addition reactions using enolates35. The presence of the trimethylsilyl group stabilizes the enolate produced and thus avoids the problems of reversibility and polymerization. Silylated ˛,ˇ-unsaturated amidate anions have also been shown to be more prone to nucleophilic attack than their unsilylated analogues36. Ordinary vinylsilanes readily undergo nucleophilic attack with strong nucleophiles such as organolithiums and this is one of the methods for generating ˛-silyl carbanions37. This has been used by Auner to make silenes38.
t Bu−Li |
H2 C |
|
CH |
|
Si |
|
Cl |
|
But |
|
CH2 |
|
CH |
|
SiCl2 |
|
|
|
|
|
|||||||||||
|
|
|
|
|
|||||||||||
|
|
|
|
|
Cl2 |
|
|
|
|
|
|
|
|
||
