
новая папка / Operative Standards for Cancer Surgery Volume I 1st Edition
.pdf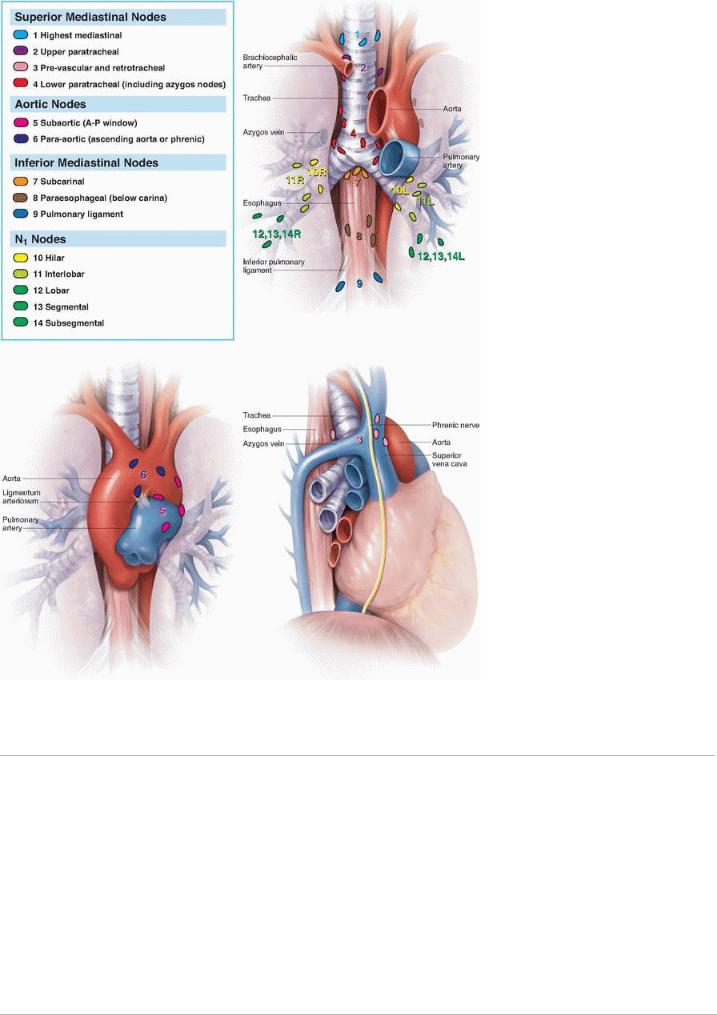
FIGURE I-3 Mountain-Dresler lymph node map. Adapted from Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-1723, with permission.
P.89
Malignant effusions (pleural and pericardial), separate tumor nodules in the contralateral lung, and pleural nodules are considered M1a. More distant spread of disease is considered M1b.
N descriptors remain the same as in the prior edition.
REFERENCES
1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62(1):10-29.
2.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). Bethesda, MD: National Cancer Institute; 2012.
3.Tieu B, Schipper P. Specialty matters in the treatment of lung cancer. Semin Thorac Surg 2012;24:99-
4.Detterbeck FC, Lewis S, Diekemper R, et al. Executive summary: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest
2013;143(5)(suppl):7S-37S.
5. Mainz J. Defining and classifying clinical indicators for quality improvement. Int J Qual Health Care
2003;15(6):523-530.
6.Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
7.Ramnath N, Dilling T, Harris L, et al. Treatment of stage III non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5)(suppl):e314S-e340S.
8.Lamb BW, Brown K, Nagpal K, et al. Quality of care management decisions by multidisciplinary cancer teams: a systematic review. Ann Surg Oncol 2011;18(8):2116-2125.
9.Lamb BW, Sevdalis N, Benn J, et al. Multidisciplinary cancer team meeting structure and treatment decisions: a prospective correlational study. Ann Surg Oncol 2013;20(3):715-722.
10.Lamb BW, Taylor C, Lamb JN, et al. Facilitators and barriers to teamworking and patient centeredness in multidisciplinary cancer teams: findings of a national study. Ann Surg Oncol 2013;20(5):1408-1416.
11.Lamb BW, Sevdalis N, Taylor C, et al. Multidisciplinary team working across different tumour types: analysis of a national survey. Ann Oncol 2012;23:1293-1300.
12.Silvestri GA, Gonzalez AV, Jantz M, et al. Methods of staging for non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidencebased clinical practice guidelines. Chest 2013;143(5)(suppl):e211S-e250S.
13.De Leyn P, Lardinois D, Van Schil P, et al. ESTS guidelines for preoperative lymph node staging for nonsmall cell lung cancer. Eur J Cardiothorac Surg 2007;32(1):1-8.
14.Detterbeck F. Integration of mediastinal staging techniques for lung cancer. Semin Thorac Cardiovasc Surg 2007;19(3):217-224.
15.Gaer JAR, Goldstraw P. Intraoperative assessment of nodal staging at thoracotomy for carcinoma of the bronchus. Eur J Cardiovasc Thorac Surg 1990;4:207-210.
16.Wu Y, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-

small cell lung cancer. Lung Cancer 2002;36(1):1-6.
17.Allen MS, Darling GE, Pechet TTV, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81(3):1013-1020.
18.Robinson L, Ruckdeschel J, Wagner HJ, et al. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based guidelines, 2nd ed. Chest 2007;132(3)(suppl):243S-265S.
19.Scott W, Howington J, Feigenberg S, et al. Treatment of non-small cell lung cancer-stage I & II: ACCP Evidence-based clinical practice guideline, 2nd ed. Chest 2007;132(3)(suppl):234S-242S.
20.Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in nonsmall cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-792.
21.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-271.
22.Veeramachaneni NK, Feins RH, Stephenson BJ, et al. Management of stage IIIa non-small cell lung cancer by thoracic surgeons in North America. Ann Thorac Surg 2012;94(3):922-926.

Chapter 5
Invasive Mediastinal Staging Overview
CRITICAL ELEMENTS
 Confirmation of Imaging Findings
Confirmation of Imaging Findings
 Mediastinal Staging for Central Tumors
Mediastinal Staging for Central Tumors
 Mediastinal Staging Prior to Treatment
Mediastinal Staging Prior to Treatment
 Mediastinal Staging at the Time of Lung Resection
Mediastinal Staging at the Time of Lung Resection
1. MEDIASTINAL STAGING FOR PATIENTS WITH SUSPECTED MEDIASTINAL NODAL INVOLVEMENT ON IMAGING
Recommendation: In general, for patients with known or suspected non-small cell lung cancer (NSCLC) who are potential surgical candidates, any positron emission tomography (PET) or computed tomography (CT) findings that suggest mediastinal nodal involvement should be confirmed with invasive mediastinal staging.
Type of Data: Retrospective.
Strength of Recommendation: Strong.
Rationale
Although PET and CT are critical components of the noninvasive clinical staging of NSCLC, both have surprisingly high false positive and false negative rates. Certain clinical presentations (e.g., granulomatous disease) can confound the interpretation of these imaging studies, which could result in inappropriate clinical stage assignment and improper care. Thus, guidelines for performing invasive staging of the mediastinum for NSCLC have been devised. More precise pretreatment staging should improve the care and survival of NSCLC patients.
P.91
Most guidelines for invasive mediastinal staging incorporate radiographic staging results, clinical T status, tumor location (central or peripheral), and available institutional technical expertise. Moreover, if PET or CT findings suggest the presence of disease in the hilum but not the mediastinum, invasive mediastinal staging is also recommended because of a substantial incidence of false negative radiographic mediastinal staging in such patients. If PET and CT results are discordant, invasive staging should be performed.
Invasive mediastinal staging techniques include cervical mediastinoscopy, endobronchial ultrasonography (EBUS), endoscopic ultrasonography (EUS), video-assisted thoracic surgery (VATS), and possibly transcervical extended mediastinal lymphadenectomy. The goal of these strategies is to accurately establish the pathologic disease stage to avoid unnecessary lung resection in patients with advanced disease and offer life-extending operations to patients whose clinical disease stage would otherwise be erroneously thought to be advanced because of false positive imaging results.
The negative predictive value of CT alone for mediastinal staging is 70% to 95% and that of PET alone is 80% to 99%. The combination of these two modalities has a negative predictive value of about 95%, indicating that in the absence of other indications, mediastinal staging is not necessary if both PET and CT reveal the mediastinal nodes to
be normal.1 CT or PET findings suggestive of abnormal mediastinal nodes in areas in which fungal or other granulomatous diseases are endemic creates challenges to accurate clinical decision making. Certainly, these findings may not represent tumor involvement of the nodes, and the imperative to document pathologic nodal status is even stronger in these instances.
2. MEDIASTINAL STAGING FOR CENTRAL TUMORS, CLINICAL N1 DISEASE, AND

LARGER TUMORS IN THE ABSENCE OF MEDIASTINAL NODAL ABNORMALITIES ON COMPUTED TOMOGRAPHY AND POSITRON EMISSION TOMOGRAPHY
Recommendation: Patients with central tumors, regardless of T status or PET or CT findings, and patients with T2 or greater disease should undergo invasive mediastinal staging.
Type of Data: Retrospective.
Strength of Recommendation: Weak.
Rationale
For patients for whom PET and CT findings are negative for mediastinal and hilar (and systemic) disease, the use of invasive lymph node staging is predicated on the T status, the location of the primary lesion, and the characteristics of ipsilateral hilar nodes on clinical staging. Patients with clinical T1a/T1b peripheral lesions (stage IA disease) and no
evidence for hilar or mediastinal adenopathy on PET and CT do not require additional mediastinal staging.2 However, patients with T2 or greater primary
P.92 tumors, clinical evidence of hilar nodal involvement, or central tumors should undergo invasive mediastinal staging,
even if their PET and CT findings are negative for disease in the mediastinum.3
There are some cogent arguments for offering invasive mediastinal staging to patients with negative PET and CT findings in the mediastinum who present with synchronous small lesions (T1a/T1b), regardless of whether these lesions are in the same lobe, in the same lung, or in opposite lungs. Also, patients for whom stereotactic body radiosurgery is planned should be subject to the same criteria as operative candidates. Local therapy with stereotactic radiosurgery may be of little value in the setting of nodal disease.
3. MEDIASTINAL STAGING PRIOR TO LUNG RESECTION
Recommendation: Proper mediastinal node staging requires a thorough understanding of the limitations of mediastinoscopy, EUS, EBUS, and VATS.
Type of Data: Retrospective.
Strength of Recommendation: Weak.
Rationale
The appropriate extent of mediastinal staging to some degree depends on the minimally invasive technique that is
used, as this determines the accessibility of the nodes.1 For traditional staging mediastinoscopy, sampled nodes should routinely include R4, L4, and 7 nodes. More stringent recommendations require that R2 and L2 specimens be obtained for all patients.
The different staging techniques afford variable access to the different lymph node stations. EBUS techniques have good lymph node yield when the nodes are enlarged and can access many level 10 and some level 11 nodes. EUS, on the other hand, offers access that is typically limited to level 7, 8, and 9 nodes. Thus, EUS can be used to diagnose the presence of mediastinal nodal involvement but is insufficient as a single staging modality to map the mediastinum. VATS typically permits access to only level 7 and ipsilateral nodes and so is useful for accessing nodes specifically targeted by PET or CT and for completing the staging of stations 5, 6, 8, and 9 when indicated. Cervical mediastinoscopy has been long held to be the gold standard of mediastinal lymph node staging and provides access to stations 2, 4, and 7 but not 5, 6, 8, or 9 nodal stations.
The first invasive modality performed for mediastinal staging should be bronchoscopic staging with EBUS, as it has a
satisfactory lymph node yield, costs less, and has a lower complication risk than mediastinoscopy or VATS does.4,5 If
abnormal inferior mediastinal adenopathy is present, EUS may be performed initially or instead of EBUS6 if institutional expertise is present. If it is not, mediastinoscopy should be performed first. The negative predictive value
of mediastinoscopy is more than 90%, and the incidence of nodal involvement detected using the modality approaches 40%
P.93
overall. However, this latter value heavily depends on the indications for mediastinoscopy.7 For example, in patients with clinical stage I disease, the incidence of nodal involvement detected by mediastinoscopy is only 3% and that
detected by thoracotomy is only 5.6%.2
Newer staging modalities such as EBUS and EUS are dependent on ultrasound localization of lymph nodes. In EBUSguided transbronchial needle aspiration, nodal biopsies are usually not attempted if the nodes cannot be visualized using ultrasonography, so these stations are often considered clinically negative. Nodes not visualized on ultrasonography are highly likely to be benign, and EBUS has a very low false negative rate for detecting nonbiopsied
“normal” nodes.8 In contrast, EUS has a false negative rate of 13% when the nodes are radiographically normal; however, this rate is higher—about 23%—in patients with other mediastinal nodal involvement. This indicates that mediastinoscopy or VATS should be used to complete mediastinal staging believed to be incomplete using EBUS alone or EBUS combined with EUS. (Please see Chapter 6 on the controversies of EBUS/EUS.) Comparative studies have demonstrated that mediastinoscopy offers a greater number of nodes sampled, a greater number of stations
sampled, and more conclusive findings than EBUS or EUS.9,10,11 Documentation of ipsilateral N2 disease alone is often insufficient to inform treatment recommendations; the status of the contralateral nodes must also be documented to enable surgeons to make specific recommendations regarding possible resection after induction therapy.
4. MEDIASTINAL STAGING AT THE TIME OF LUNG RESECTION
Recommendation: At the time of lung resection, on the right side, sampled/dissected nodes should include R10, R9, R8, 7, R4, and R2 nodes. On the left side, sampled/dissected nodes should include L10, L9, L8, 7, 6, 5, and L4 nodes and L2 nodes if accessible.
Type of Data: Retrospective.
Strength of Recommendation: Weak.
Rationale
The hilum and mediastinum should be thoroughly staged at the time of lung resection, even in patients who are undergoing nonanatomic parenchyma-sparing resections such as segmentectomy or wedge resection. There is no conclusive evidence that nodal dissection provides more complete staging information or results in better outcomes than nodal sampling does. On the right side, the sampled or dissected nodes should include the R9, R8, 7, R10, R4, and R2 nodes. On the left side, the sampled or dissected nodes should include the L9, L8, 7, 6, 5, and L4 nodes and the L2 nodes if accessible. Preservation of the recurrent laryngeal nerve takes precedence over complete nodal dissection in station 5.
P.94
P.95
TABLE 5-1 Literature Review
|
|
|
No. of |
|
|
Potential |
Author |
Year |
Study Design |
Patients |
Main Question |
Key Findings |
for Bias |
Groth1 |
2008 |
Literature review |
N/A |
How accurate is |
The accuracy of |
No |
|
|
|
|
radiographic |
PET and/or CT |
|
|
|
|
|
mediastinal |
is not as good |
|
|
|
|
|
staging for |
as that of |
|
|
|
|
|
|
|
|

|
|
|
|
NSLCL? |
invasive |
|
|
|
|
|
|
mediastinal |
|
|
|
|
|
|
staging. |
|
Schipper8 |
2008 |
Literature review |
N/A |
What is the |
No conclusive |
No |
|
|
|
|
comparative |
outcomes |
|
|
|
|
|
accuracy of |
|
|
|
|
|
|
minimally |
|
|
|
|
|
|
invasive to |
|
|
|
|
|
|
invasive |
|
|
|
|
|
|
techniques for |
|
|
|
|
|
|
mediastinal |
|
|
|
|
|
|
nodal staging in |
|
|
|
|
|
|
NSCLC? |
|
|
Meyers2 |
2006 |
Retrospective |
248 |
What is the |
The incidence of |
No |
|
|
review of single |
|
incidence of |
occult nodal |
|
|
|
institution |
|
occult |
metastases was |
|
|
|
patient cohort |
|
mediastinal |
5.6%. |
|
|
|
|
|
nodal |
|
|
|
|
|
|
involvement in |
|
|
|
|
|
|
patients with |
|
|
|
|
|
|
clinical stage I |
|
|
|
|
|
|
NSCLC? |
|
|
Licht12 |
2013 |
Retrospective |
1513 |
What is the |
The incidence of |
No |
|
|
review of |
|
incidence of |
occult nodal |
|
|
|
national |
|
unsuspected |
metastases was |
|
|
|
database |
|
mediastinal |
7.9%. |
|
|
|
|
|
nodal disease in |
|
|
|
|
|
|
clinical stage I |
|
|
|
|
|
|
NSCLC? |
|
|
Crabtree13 |
2010 |
Retrospective |
462 |
What is the |
The incidence of |
No |
|
|
single institution |
|
incidence of |
occult nodal |
|
|
|
patient cohort |
|
unsuspected |
metastases was |
|
|
|
|
|
nodal disease in |
3.5%. |
|
|
|
|
|
clinical stage IA |
|
|
|
|
|
|
NSCLC? |
|
|
Zhang9 |
2012 |
Prospective |
36 |
What is the |
EBUS has a |
Yes— |
|
|
observational |
|
relative |
lower diagnostic |
EBUS |
|
|
study of patients |
|
accuracy of |
yield and |
operator |
|
|
undergoing both |
|
mediastinoscopy |
resulted in |
skill not |
|
|
EBUS and |
|
compared to |
mediastinal |
assessed |
|
|
mediastinoscopy |
|
EBUS for |
understaging in |
|
|
|
to stage NSCLC |
|
assessing |
patients with |
|
|
|
|
|
mediastinal |
NSCLC. |
|
|
|
|
|
nodes? |
|
|
Smulders10 |
2005 |
Retrospective |
156 |
What was the |
Absence of |
No |
|
|
cohort study |
|
accuracy of |
mediastinal |
|

|
|
|
|
mediastinoscopy |
node |
|
|
|
|
|
in staging |
involvement was |
|
|
|
|
|
mediastinal |
correctly |
|
|
|
|
|
nodes in |
identified in |
|
|
|
|
|
patients with |
93.6%. |
|
|
|
|
|
NSCLC? |
|
|
Annema11 |
2005 |
Prospective |
242 |
What is the |
EBUS |
No |
|
|
observational |
|
impact of EBUS |
eliminated 70% |
|
|
|
study |
|
on the routine |
of surgical |
|
|
|
|
|
use of |
procedures. |
|
|
|
|
|
mediastinoscopy |
|
|
|
|
|
|
or thoracotomy |
|
|
|
|
|
|
for potentially |
|
|
|
|
|
|
resectable |
|
|
|
|
|
|
NSCLC? |
|
|
Silvestri4 |
2013 |
Literature review |
N/A |
What is the |
EBUS (and |
No |
|
|
and consensus |
|
optimal |
EUS) is the first |
|
|
|
process |
|
algorithm for |
choice for |
|
|
|
|
|
invasive |
invasive |
|
|
|
|
|
mediastinal |
mediastinal |
|
|
|
|
|
staging? |
staging. If |
|
|
|
|
|
|
negative, these |
|
|
|
|
|
|
techniques |
|
|
|
|
|
|
should be |
|
|
|
|
|
|
followed by |
|
|
|
|
|
|
surgical biopsy. |
|
ASGE6 |
2011 |
Literature review |
N/A |
What is the |
EUS has utility |
No |
|
|
and practice |
|
utility of EUS in |
for staging lower |
|
|
|
guidelines |
|
staging the |
mediastinal and |
|
|
|
statement |
|
mediastinum in |
level 5 nodes. |
|
|
|
|
|
patients with |
|
|
|
|
|
|
NSCLC? |
|
|
ESTS5 |
2014 |
Unpublished |
|
|
|
|
Toloza7 |
2003 |
Systematic |
N/A |
What are |
TBNA sensitivity |
No |
|
|
review and |
|
performance |
0.76, NPV 0.71. |
|
|
|
meta-analysis |
|
characteristics |
EUS sensitivity |
|
|
|
|
|
of invasive |
0.88, NPV 0.77. |
|
|
|
|
|
mediastinal |
Mediastinoscopy |
|
|
|
|
|
staging |
sensitivity 0.81, |
|
|
|
|
|
modalities? |
NPV 0.91. |
|
CT, computed tomography; EBUS, endobronchial ultrasonography; EUS, endoscopic ultrasonography; NPV, negative predictive value; NSCLC, non-small cell lung cancer; PET, positron emission tomography; TBNA, transbronchial needle aspiration.

P.96
REFERENCES
1.Groth SS, Whitson BA, Maddaus MA. Radiographic staging of mediastinal lymph nodes in nonsmall cell lung cancer patients. Thorac Surg Clin 2008;18(4):349-361.
2.Meyers BF, Haddad F, Siegel BA, et al. Cost-effectiveness of routine mediastinoscopy in computed tomographyand positron emission tomography-screened patients with stage I lung cancer. J Thorac Cardiovasc Surg 2006;131(4):822-829; discussion 822-829.
3.De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardio-Thorac Surg 2014;45:787-798.
4.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5)(suppl):e211S-e250S.
5.DeLeyn P, Dooms C, Kuzdzal J et al. Revised ESTS guidelines for preoperative mediastinal lyph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg 2012;45(5):787-798.
6.ASGE Standards of Practice Committee; Jue TL, Sharaf RN, Appalaneni V, et al. Role of EUS for the evaluation of mediastinal adenopathy. Gastrointest Endosc 2011;74(2):239-245.
7.Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123(1)(suppl):137S-146S.
8.Schipper P, Schoolfield M. Minimally invasive staging of N2 disease: endobronchial ultrasound/transesophageal endoscopic ultrasound, mediastinoscopy, and thoracoscopy. Thorac Surg Clin 2008;18(4):363-379.
9.Zhang R, Mietchen C, Krüger M, et al. Endobronchial ultrasound guided fine needle aspiration versus transcervical mediastinoscopy in nodal staging of non-small cell lung cancer: a prospective comparison study. J Cardiothorac Surg 2012;7:51.
10.Smulders SA, Smeenk FW, Janssen-Heijnen ML, et al. Surgical mediastinal staging in daily practice. Lung Cancer 2005;47(2):243-251.
11.Annema JT, Versteegh MI, Veseliç M, et al. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of lung cancer and its impact on surgical staging. J Clin Oncol 2005;23(33):8357-8361.
12.Licht PB, Jørgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96(3): 943-949; discussion 949-950.
13.Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140(2):377-386.
Chapter 6
Endobronchial Ultrasonography/Endoscopic Ultrasonography
CRITICAL ELEMENTS
 Identification of Lymph Nodes Suspicious for Cancer Metastasis
Identification of Lymph Nodes Suspicious for Cancer Metastasis
 Node Station Assessment Utilizing Endobronchial Ultrasonography/Endoscopic Ultrasonography
Node Station Assessment Utilizing Endobronchial Ultrasonography/Endoscopic Ultrasonography
1. IDENTIFICATION OF LYMPH NODES SUSPICIOUS FOR CANCER METASTASIS
Recommendation: The standard endobronchial ultrasonography (EBUS) classification system of the sonographic features of lymph nodes is useful to determine whether lymph nodes are malignant or benign.
Type of Data: Retrospective.
Strength of Recommendation: Weak.
Rationale
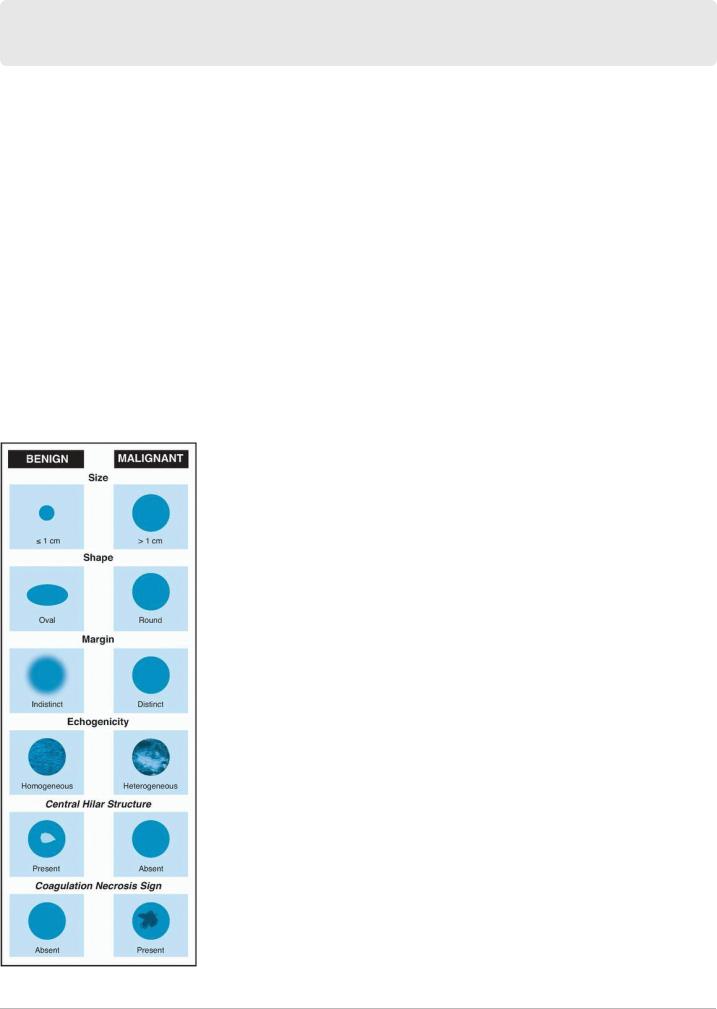
Round lymph nodes whose short-axis diameter is larger than 1 cm, that have distinct margins and heterogeneous echogenicity, and that have
coagulation necrosis sign but not central hilar structures are suspicious for malignancy and must be biopsied (Fig. 6-1).1
During EBUS-guided transbronchial needle aspiration (EBUS-TBNA) or endoscopic ultrasonography (EUS)-guided fine needle aspiration (EUSFNA), all mediastinal and hilar lymph nodes should be assessed, characterized, and documented systematically. Lymph nodes should be
identified according to the International Association for the Study of Lung Cancer lymph node map (Fig. I-3).2 EBUS-TBNA and EUS-FNA should proceed from N3 nodes to N2 nodes and then to N1 nodes to prevent needle contamination and avoid accidental disease overstaging.
P.98
FIGURE 6-1 Sonographic characteristics, by EBUS or EUS, of mediastinal lymph nodes that favor benignancy or malignancy in lung cancer.
P.99 Morphology ultrasonography should be used to assess mediastinal lymph node stations 2R, 4R, 2L, 4L, and 7 during EBUS and stations 8 and 9
