
Учебное пособие 2210
.pdf
Issue № 4 (36), 2017 |
ISSN 2542-0526 |
anionite. There is some water in it due to hydration of the functional groups that are positively charged (see Fig. 1). A decrease in the moisture content indicates a drop in their amount. This indirect fact proves the data from determining the total volumetric capacity of the anionite before and after it was sifted through water (Fig. 3).
Exchange capacity, mg-equiv/g
Fig. 3. Change in the total exchange capacity (1) and amount of strongly dissociated functional groups (2) depending on the number
of regenerations
Number of regenerations using the alkaline solution
Note that the difference between the total exchange capacity and the number of strongly ionized groups is that of the low-base, i.e. weakly dissociated groups. It is 0.72…0.75 mgequiv/l in the sample for sale (3.89––3.15) as well as in the other portions of the anionite. Thus during filtration of water anionite loses only strong-based functional groups. As a result, the total exchange capacity drops. I.e. there is deaminization of the anionite (a decrease in the amount of nitrogen-containing fragments (Fig. 1)) [18]:
R-CH2N (CH3)3OH + H2O R-CH2OH + NH (CH3)3OH.
Since the destruction products are constantly removed from the ionite, the balance of the reaction constantly moves to the right, i.e. to its deaminization. How long is this process going to occur? This is a very important question as the substances washed out of the anionite АV-17-8 pollute the purified water at the final stage of desalination.
The obtained results directly indicate a constant removal of the impurities from the ionexchangers even in distilled water. In the “dummy” experiment the total exchange capacity dropped from 3.15 to 2.34 mg-equiv/g, i.e. these indices are absolutely the same as for the experiment with 50 regenerations when 100000 volumes of water were sifted. The only difference is that the proportion of the destroyed granules is not over 14.2 % compared to 34.0 % in the experiments with the regeneration of the anionite.
I.e. we can safely say that in the dynamic mode 14 % of the anionite is destroyed simply by a water flow and the treatment with the alkaline solution causes another 20 % of the loading to
81

Russian Journal of Building Construction and Architecture
crack. This is inevitable since as the alkaline solution comes into the filter, the anionite grain shrinks according to the osmosis law as well as the anionite is converted from the saline (a carbon-dioxide, poorly swelling form) into the hydroxide high-water ionic form causing the volume of the grain to increase.
During the design of the above GOST 16187-70 the recommemded treatment was considered sufficient as there was no data regarding the behavior of ionites during water desalination. Later on, when some better-quality water was becessary to produce large intergral schemes, the problem of preliminary preparation of the ion-exchangers emerged once again.
4. Studies of the efficiency of different ways of purification commenced many years ago [17]. Deep washing of ionites from impurities was performed using a Soxhlet extractor [9]. A solvent is poured into a flask. At the mouth of the flask there is a glass tube with a sealed porous partition with an ionite. As the flask heats up, the water steam goes through the weighed portion of the ionite and comes into a reflux condenser where it is condensed with the resulting water flowing into the flask. Therefore there is multiple washing of the ionite with the solvent steam with the same amount of water being evaporated. After 20 hours of the extraction, the solvent was replaced.
The samples for sale (original) of ionites were tested (as a comparison item) as well as those prepared in two ways: in accordance with the GOST 16187-70 (method I) and following the treatment of the ionite with a 1.5 mole/l solution of hydrochloric acid washing with water with a 0.5 mole/l solution of caustic soda and water again (method II) [9].
The way the sample is treated appeared to impact the way the ionite is washed from impurities in the extractor. The total amount of the reducing impurities extracted from the cationite КU-2-8 is shown in Fig. 4.
Oxidability, O2/l
b)
|
|
|
|
|
Fig. 4. The total amount of the substances washed |
a) |
|
|
|
||
|
|
|
out of the cationite КU-2-8 after 100 hours of the |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
steam extraction using permanganate (а) |
|
|
|
|
|
and bichromate (b) oxidability |
|
|
|
|
|
(methods I and II) |
|
|
|
|
|
|
|
Original |
|
Original |
|
|
|
|
|
|
|
|
82

Issue № 4 (36), 2017 |
ISSN 2542-0526 |
Based on the data obtained in [9], the sample for sale (original) produce less water pollution, i.e. more slowly than the prepared ones. This means that they will pollute the water for infinitely long. It might be due to the fact that during the treatment of the anionite using the method I the type (NaCI, NaOH, HCI) and concentration of the reagents changed as well as the ionic form of the cationite, which caused the granules to shrink or expand. This led the ionite “to breathe”, which made it easier for the impurities to come out of the opened pores. It is clearly seen that an emission of the substances is larger from the sample prepared using the method I that was kept for even swelling in the concentrated solution of NaCI. A change in the amount of the washed organic substances in the oxidability units along the cycles is shown in Fig. 5.
Oxidability, mg O2/l
Number of washing cycles
Fig. 5. Change in the biochromate (1) and permanganate (2) oxidability of the extracts
According to the position and form of the curved lines in Fig. 5, the oxidability indices dropped sharply over the first four extraction cycles. Further on, the result did not change much, which makes it unreasonable to continue the washing. This is proved by the results of the objective identification of the optical density of the extracts in all of the cycles measured at the wavelength of 215 nm.
It can be concluded that there is constantly some amount of reducing compounds coming out of the cationite КU-2-8. This is considered as solubility, or the result of the destruction of the cationite.
The comparison of the optical density of the extracts from the cationite КU-2-8 and anionite АV-17-8 allows one to conclude that the cationite has a lower chemical stability, which is indicated by higher scores on this test (Fig. 6).
These ion-exchangers are synthesized using the same matrix – an interpolymer of styrene and divinylbenzene. What is different is the type of a carrier of functional groups: they are introduced into the cationite by means of sulfonating and the anionite using amination. There might be polluting substances coming into the ionites or forming there at this stage.
83

Russian Journal of Building Construction and Architecture
Optical density |
Distillate |
|
|
Number of washing cycles
Fig. 6. Curved lines of washing of the anionite АV-17-8 from organic impurities (red) and cationite КU-2-8 (blue)
in the cycles 1—5 along the value of the optical density of the fractions along the wavelength of 240 nm
The solvents, i.e. dioxane and benzene, were used while searching for an effective way of washing the ionites. In this series of the experiments extraction was performed over 100 hours. One cycle was 20 hours. 50 ml of the ionite and 100 ml of the solvent was used. After 20 hours the amount of the solvent was changed. The extracts were analyzed and the results of measuring their optical density are given in Fig. 7.
Optical density
Fig. 7. Absorption spectra of pure dioxane in a bidistillate (1) and dioxane extracts from the cationite КU-2-8 of the sample for sale (2) and the one treated using the method I (3)
Wavelength, nm
As shown in Fig. 7, the dioxane as well as the water steam extracts the impurities from the ionite. As a result, the optical density of the solvent rises both following the contact with the sample for sale as well as with a preliminarily prepared sample of the cationite КU-2-8. Note that the same dependence is found in the investigation of the benzene extracts with the absorption maximum being not at 240 but at 250 nm.
Therefore treatment of the ionites using benzene and dioxane also failed to prevent the impurities from washing away. Ion-exchanging materials seem to constantly pollute purified water and their pure forms are impossible to obtain [9]. This misfortunate fact is concluded based on the results of the study [25].
We have investigated the influence of the multiplicity of interchangeable treatment of the anionites АV-17-2P and АV-17-10P with the solutions of КОН and НС1 per the amount of
84
Issue № 4 (36), 2017 |
ISSN 2542-0526 |
the sifted distilled water that was estimated according to its optical density at = 205 nm and the length of the ditch of 100 mm as well as the permanganate oxidability of the washed water and regenerates [25].
We might have been able to free these two samples of the anionite exchangers from impurities completely. We concluded that based on the fact that as they were held in distilled water for a long time, their optical density did not increase. However, stopping the non-polymerized monomers and other side products from being extracted became possible by means of a huge consumption of the reagents and water: in the laboratory setting 50 equiv. of NaOH, 30 equiv. of HCl and 900 fold volumes of a distillate per 1 l of the ionite was consumed [25]. The use of the sorbents prepared in such a way for water desalination revealed that the anionites do not emit but, on the contrary, absorb its organic substances with the optical desnity dropping from 0.39 to 0,14 (АV-17-10P) and from 0,39 to 0,075 (АV-17-2P). However, this consumption is a source of a plethora of environmental issues. Thus technology experts seek to perform preliminary treatment of ionites in a more environmentally-friendly manner.
The authors [3] concluded: there are ionites that emit biologically active impurities into water whatever washing method is used. These are anionite АNT-251 based on divinylbenzene and 2-methyl-vinylpyridine. They cannot be used in the technologies that process water contact food products. They emit phenol (КU-1) into water, formaldehyde (anionites based on the anionite IИА-2), plastic polyamines (anionites АV-16GS, EDE-10P, АN-31, etc.) The amount of the emitted monomers is more than the maximum permissible concentration.
The chemical stability of ionites in the above studies [12, 14, 25] was investigated in water solutions. The authors [7] explored the behavior of ionites in sugar solutions as they were used to remove the coloring substances in the refined sugar production at a high temperature. The extract is obtained using ionites: 1 volume of the ionite and 7 volumes of water held for 1 and 8 hours at the temperature of 80 оС. Besides the oxidability, the bromine absorption number (non-specific compounds) and solid residual (fixed substances). All these groups of the impurities were found in the extracts. IR-spectra indicate that there are fragments of ionexchangers. I.e. the ionites based on styrene are partially converted into the soluble condition in water as well as the sugar solutions. According to [7], they are mostly extracted from the parties of the ionites that are in use in industrial filters.
The latter data was of interest to us as in ion-exchanging setups of desalination ionexchangers are operated for years. The influence of an operating cationite on the composition of water impurities was investigated using an industrial ion-exchanging setups of desalination of river water with the productivity of 40 m3/h.
85

Russian Journal of Building Construction and Architecture
Over the entire operating period water samples were selected before and after a filter with the cationite КU-2-8. The content of humic and fulvic acids that are invariably found in natural water and yield a yellowish coloring was determined. The results of the analysis of the samples are presented in Table.
Table
Change in the quality of river water during its filtration through the cationite КU-2-8
The |
Content of fulvic acids, |
Increase in the |
Content of humic acids, |
Increase in |
|||
amount of |
50mkg/l |
|
10 mkg/dm3 |
||||
|
СFA, |
the СHA, |
|||||
the sifted |
|
|
|
|
|
||
Before the |
|
|
Before the |
|
|||
|
After it |
mkg/l |
After it |
mkg/l |
|||
water, m3 |
cationite |
|
cationite |
||||
|
|
|
|
|
|||
|
|
|
|
|
|
270 |
|
80 |
860 |
|
200 |
660 |
70 |
200 |
|
|
|
|
|
|
|
300 |
|
400 |
300 |
|
1300 |
1000 |
140 |
160 |
|
|
|
|
|
|
|
190 |
|
1000 |
850 |
|
1750 |
900 |
60 |
130 |
|
|
|
|
|
|
|
160 |
|
1600 |
880 |
|
1040 |
160 |
80 |
80 |
|
|
|
|
|
|
|
75 |
|
1800 |
910 |
|
1030 |
120 |
50 |
25 |
|
|
|
|
|
|
|
|
|
Based on the data, we conclude that during the entire operating period the concentration of humic СHA and fulvic acids СFA in Н-cationed water is larger than in the original water even at the end of the filtration cycle. But their content in the filtrate decreases over time, i.e. the cationite “is washed away” from organic impurities in a way that are desorbed during oxide washing, which is rather unexpected. Cationites that have negatively charged functional groups ( SO3-) are considered to be incapable of absorbing these organic groups. However, this experiment (table) state the contrary.
5. Microbiological pollution of filtrated water was identified in electronic industry enterprises. One of the requirements to the water quality is that there are no solid particles including microorganisms.
The analysis of desalinated water showed [10] that bacteria grow most actively on the anionite filter, which, we think, is easily explained by the fact that there are aminogroups in its structure whose nitrogen is a biogenic element, i.e. it is necessary to sustain the lives of other organisms. In addition, humic and fulvic acids containing nitrogen are absorbed on the granules of anionite during water treatment. This contributes to a rapid growth of microorganisms in water.
A significant growth of the number of bacteria is typical of isolated contours [16]. Tehse systems occur in the cooling cycles of electrophysical tools. They are filled with desalinated wa-
86
Issue № 4 (36), 2017 |
ISSN 2542-0526 |
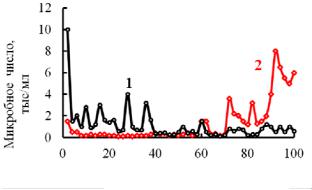
ter. It circulates through the contour and column with the ionites: the cationite КU-2-8 and anionite АV-17-8. The results of the evaluation of the number of bacteria are in Fig. 8.
number ofmicrobes, |
thousand/ml |
The |
|
|
|
Time, h
Fig. 8. Сontent of microorganisms at the input (1) and output of the column (2)
There is an interesting dependence here: originally the amount of microbes was smaller than that for the incoming water. They it becomes different (40…70 hours of operation). In some time there is more microflora in the filtrated than in the original water. Note that the amount of microorganisms was determined using the sifter method, i.e. by applying an aliquot of water onto a substantial medium.
Such a growth in the amount of microflora is due to the fact that bacteria originally fix on the surface of the ionite granules and remain there. Then the other bacteria are absorbed forming a few layers. Their interaction energy with the solid surface is not large. If the surface is considerably filled, some of the microflora is simply washed away and end up in the water flow. Then they are replaced by other microorganisms and their amount in the water flow drops again [16]. The type of bacteria and their amount was evaluated in [15]. While controlling the upper layer of the ion-exchanger it was found that it was infested with microorganisms. Water samples were selected and 21 media was used for sifting. The amount of bacteria in the original pipe water is an average of 2…7. Following desalination their amount goes up from 13000… 24000 per 1 ml.
The type of microorganisms and the number of samples N was determined where they were identified (the total of 15). The data are presented in Fig. 9.
As we can see, the microorganisms of all types that ended up on the ion-exchange materials along with the original pipe water do not die but keep reproducing [15]. They adapt to the conditions that are formed in the filter, make up capsule forms that produce a lot of slime. It accumulates in the water pipes and around the ionite grains. The amount of impurities in water grows quickly over the entire observation period.
87

Russian Journal of Building Construction and Architecture
The data on the bacterial pollution of water during its filtration through inert materials are of interest as well. It is described in [15]. The amount of the microorganisms that emerge in the water sifted through carbon and sand is compared. Pipe water was almost free from them (2…8 bacteria in 1 ml).
Fig. 9. Change in the amount of microorganisms of pipe (blue) and desalinated water (yellow). The following microorganisms are numbered: 1 are thiobacteria; 2 arepurple bacteria; 3 are tyone bacteria; 4 are ferrobacteria; 5 are desulfurization bacteria; 6 are oil bacteria
The results of the calculation of the amount of microorganisms of different types in the original water and following the filtration are shown in Fig. 10.
Amount of microorganisms in 1 ml of water
Time of the observation, days
Fig. 10. Change in the content of microorganisms in the pipe water following the filtration through active carbon
(1) and sand (2)
The contact with both components is seen to enrich the water with microorganisms by hundreds and thousands of times. A water pollution degree is larger after carbon has been used. It is easy to explain as carbon absorbs organic impurities including nitrogen-containing ones that are nutrition for bacteria.
Therefore secondary pollution of purified water occurs when ionites of different types are used as well as inert filtration materials.
Conclusions
Synthetic ion-exchangers contain not only a matrix of interpolymers. One of the components of styrene-divinyl ionites that have ethylstyrene, ethylbenzene, styrene, xylol, diethylbenzene in them [21]. Thus some of the substances take part in the polymerization reaction with some of them remaining in the synthesized matrix and washed away by water.
88
Issue № 4 (36), 2017 |
ISSN 2542-0526 |
It is shown that most of them can be removed by means of three-fold interchangeable treatment of ionites with acid and alkaline solutions. In order to reduce the consumption of reagents, it is necessary that the concentration of reagents (compared to the mode [25]) is reduced by 3—4 times but the time ionites are held in these media are extended.
Prior to introducing filters into the operation, the first portions of water should be damped and its quality should be controlled according to its optical density. If the index is equal at the input and output, water should be further supplied according to a technological cycle.
Following the treatment it is recommended that ionites in filters are regenerated immediately after they are switched off. If one fails to do that, the motionless ionite will grow with groups of microorganisms particularly the anionite that actively absorbs humic and fulvic acids from water, i.e. nitrogen-containing organic substances that nourish the microflora. In order to prevent these substances from entering filters with anionites and cationites, water should be deeply purified from organic impurities by means of a combination of different methods prior to being supplied for desalination.
Regenerated filters should not be completely be washed from acid and alkaline as microorganisms will stop (or will be more slowly) reproducing in such a medium. The final removal of regenerates will be performed before filters are introduced into operation.
Water after filters with inert nozzles should be sterilized using ultrasound, ultraviolet radiation, boiling.
References
1.Klimenko N. A., Koganovskiy A. M., Smolin S. K. i dr. Adsorbtsionnaya ochistka rechnoy i pit'evoy vody i rol' biodegradatsii adsorbirovannykh veshchestv v etom protsesse [Adsorption purification of river and drinking water and the role of the biodegradation of adsorbed substances in the process]. Khimiya i tekhnologiya vody, 1997, vol. 19, no. 4, pp. 382—385.
2.Grafova I. A., Mel'nik L. A., Penkalo I. I. Glubokaya ochistka ionitov, ispol'zuemykh v protsessakh vodopodgotovki [Deep cleaning of ion exchange resins used in water treatment processes]. Khimiya i tekhnologiya vody, 1992, vol. 14, no. 3, pp. 185—199.
3.Grebenyuk V. D., Mazo A. A. Obessolivanie vody ionitami [Desalination of water by ionites]. Moscow, Khimiya Publ., 1980. 256 p.
4.Zambrovskaya E. V., Belyaev I. A., Shcheglov L. L. Issledovanie primesey, vydelyaemykh iz chistykh form ionitov [The study of impurities emitted from the pure forms of ion exchangers]. Khimiya i tekhnologiya vody, 1990, vol. 12, no. 19, pp. 931—934.
5.Ivanova E. V., Chikin G. A. Otsenka gigienicheskikh svoystv ionoobmennykh materialov [Assessment of hy-
gienic properties of ion-exchange materials]. Teoriya i praktika sorbtsionnykh protsessov, 1991, vol. 21, pp. 119—123.
89

Russian Journal of Building Construction and Architecture
6.Ionity: katalog [Ionity: catalog]. Cherkassy, Izd-vo NIITEKhIM, 1975. 36 p.
7.Klochkova T. A. O khimicheskoy stoykosti polimerizatsionnykh ionitov KU-2 i AV-17 [About the chemical stability of the polymerization ion exchangers KU-2 and AB-17]. Teoriya i praktika sorbtsionnykh protsessov, 1971, vol. 6, pp. 136—139.
8.Kul'skiy L. A., Strokach P. P. Tekhnologiya ochistki prirodnykh vod [The technology of purification of natural waters]. Kiev, Vishcha shkola Publ., 1986. 352 p.
9.Mazo A. A., Granovskaya G. L., Voytovich V. B. K ochistke ionitov ot postoronnikh organicheskikh primesey [Cleaning of ion exchange resins from extraneous organic impurities]. Teoriya i praktika sorbtsionnykh protsessov, 1966, vol. 1, pp. 136—141.
10.Mazo A. A., Novoseletskiy A. G. Izmenenie mikroflory vody v protsesse ee obessolivaniya ionoobmennymi smolami [Changes of the microflora of the water in the process of desalting by ion-exchange resins]. Teoriya i praktika sorbtsionnykh protsessov, 1972, vol. 4, pp. 133—137.
11.Mamchenko V. A., Vaynman A. B., Zanina G. V. Tekhnologicheskie kharakteristiki anionitov razlichnykh tipov pri obessolivanii vody [Technological characteristics of anion exchangers of various types in the desalination of water]. Khimiya i tekhnologiya vody, 1997, vol. 19, no. 4, pp. 404—416.
12.Mel'nik L. A., Grafova I. A., Penkalo I. I. Ochistka anionita AV-17 ot organicheskikh veshchestv [Purification of the anion exchanger AV-17 from organic substances]. Khimiya i tekhnologiya vody, 1990, vol. 12, no. 8, pp. 741—743.
13.Milyukin M. V., Tkachuk T. M., Klimenko N. A. [Identification of organic compounds leached from some of the sorbents]. Trudy Mezhdunarodnoy nauchno-tekhnicheskoy konferentsii «Ekologiya khimicheskikh proizvodstv» [Proc. of International scientific-technical conference "Ecology of chemical manufactures"]. Severodonetsk, 1994, pp. 33—34.
14.Moskvin L. N., Godon L. A., Epimakhova L. V. Opredelenie produktov destruktsii ionoobmennykh smol v vysokochistoy vode [Determination of degradation products of ion exchange resins in high purity water].
Vysokochistye veshchestva, 1988, no. 3, pp. 164—167.
15.Nesterova G. N., Kolmakov O. A., Aleksandrova L. K., Komarov I. I. [Microbiological contamination of deionized water]. Problemy polucheniya osobo chistoy vody [The problems of producing ultra-pure water]. Voronezh, Izd-vo VGU, 1971, pp. 88—93.
16.Novoseletskiy A. G., Demchenko S. N. Kolichestvennye izmeneniya mikroflory obessolennoy vody, tsirkuliruyushchey v zamknutom tsikle [Quantitative changes of the microflora of the desalinated water circulating in a closed loop]. Teoriya i praktika sorbtsionnykh protsessov [Theory and practice of sorption processes], 1972, vol. 7, pp. 139—141.
17.Pashkov S. I. Opyt ul'trazvukovoy ochistki ionitov [Experience of ultrasonic cleaning of ion exchangers]. Energetik, 1976, no. 3, pp. 30—31.
18.Polyanskiy N. G., Gorbunov G. V., Polyanskaya N. L. Metody issledovaniya ionitov [Research methods of ion exchangers]. Moscow, Khimiya Publ., 1976. 206 p.
19.Ryabchikov B. E. Sovremennye metody podgotovki dlya promyshlennogo i bytovogo ispol'zovaniya [Modern methods of preparation for industrial and domestic use]. Moscow, DeLi print Publ., 2004. 328 p.
20.Sal'nikov M. A., Belyaev I. A., Zambrovskaya E. V. Issledovanie kachestva ionitov, ispol'zuemykh v proizvodstve deionizovannoy vody [Study of the quality of ion exchangers used in the production of deionized water]. Elektronnaya promyshlennost', 1991, no. 4, pp. 60—61.
90
