
книга / 2016_Kaplan_USMLE_Step_1_Lecture_Notes_Pharmacology
.pdf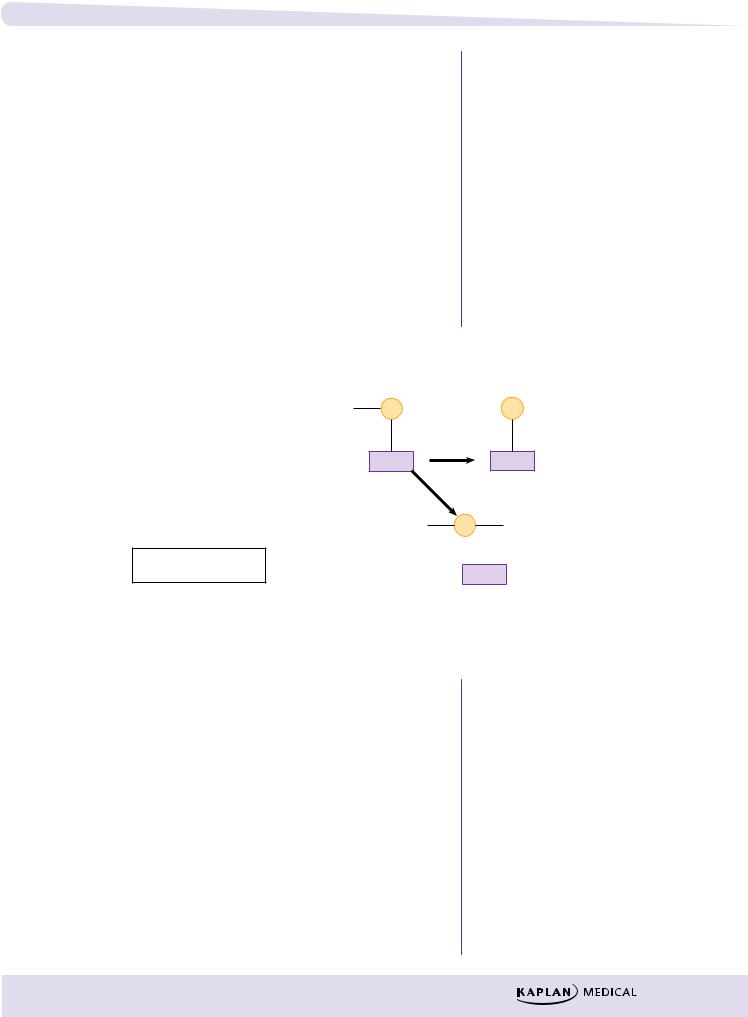
Chapter 2 λ Cholinergic Pharmacology
–Bronchoconstriction
–Lacrimation
–Salivation
–Sweating
–CNS stimulation
λNicotinic effects:
–Skeletal muscle excitation followed by paralysis
–CNS stimulation
Management
λMuscarinic effects: atropine
λRegeneration of AChE: pralidoxime (2-PAM)
λTime-dependent aging requires use of 2-PAM as soon as possible (see Figure II-2-3).
Irreversibly Acting Cholinomimetics:
These compounds phosphorylate the esteratic site on AChE, at serine hydroxyl groups
1.phosphorylation; reversible by pralidoxime (2-PAM)
2.removal of a part of the organophosphate molecule (aging); complex no longer reversible by 2-PAM
R = leaving group
P = organophosphate
|
BLOCKADE |
AGING |
R |
P |
P |
H2O
AChE AChE
2-PAM
R P 2-PAM
REGENERATION AChE
Figure II-2-3. Effects of Organophosphate on AChE
Chronic toxicity
λPeripheral neuropathy causing muscle weakness and sensory loss
λDemyelination not due to AChE inhibition
MUSCARINIC RECEPTOR ANTAGONISTS
Atropine
λPrototype of the class
λAs a tertiary amine, it enters CNS
λOther M blockers differ mainly in their pharmacokinetic properties
49
Section II λ Autonomic Pharmacology
Bridge to Physiology
ANS Dominance
For effector tissues with dual innervation, PANS is dominant. These include the SA and AV nodes of the heart, the pupil, GI and GU muscles, and sphincters. SANS is dominant only in terms of vascular tone and thermoregulatory sweat glands.
Pharmacologic effects
λAtropine effects in order of increasing dose:
–Decreased secretions (salivary, bronchiolar, sweat)
–Mydriasis and cycloplegia
–Hyperthermia (with resulting vasodilation)
–Tachycardia
–Sedation
–Urinary retention and constipation
–Behavioral: excitation and hallucinations
λOther classes of drugs with antimuscarinic pharmacology:
–Antihistamines
–Tricyclic antidepressants
–Antipsychotics
–Quinidine
–Amantadine
–Meperidine
λTreatment of acute intoxication:
–Symptomatic ± physostigmine
Table II-2-6. Clinical Uses and/or Characteristics of M Blockers
Drug |
|
Clinical Uses and/or Characteristics |
Atropine |
|
Antispasmodic, antisecretory, management of |
|
|
AChE inhibitor OD, antidiarrheal, ophthalmology |
|
|
(but long action) |
Tropicamide |
|
Ophthalmology (topical) |
Ipratropium, |
|
Asthma and COPD (inhalational)—no CNS entry, |
tiotropium |
|
no change in mucus viscosity |
Scopolamine |
|
Used in motion sickness, causes sedation and |
|
|
short-term memory block |
Benztropine, |
|
Lipid-soluble (CNS entry) used in parkinsonism |
trihexyphenidyl |
|
and in acute extrapyramidal symptoms induced |
|
|
by antipsychotics |
Oxybutynin |
|
Used in overactive bladder (urge incontinence) |
NICOTINIC RECEPTOR ANTAGONISTS
Ganglion Blocking Agents
λDrugs: hexamethonium and mecamylamine
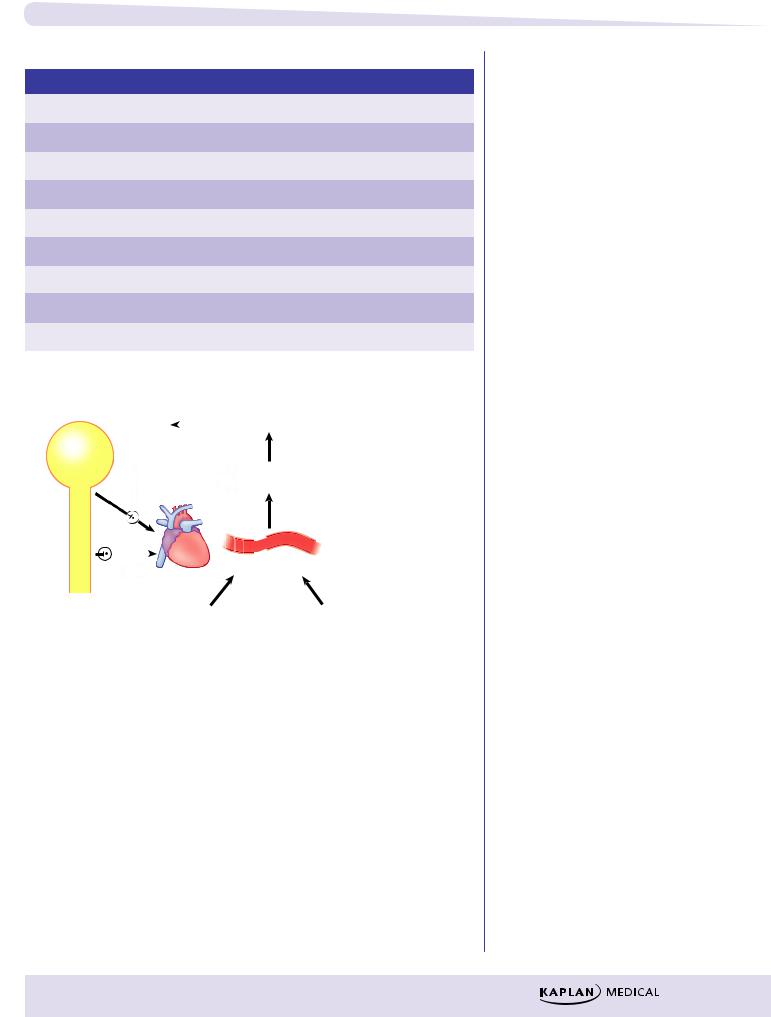
λReduce the predominant autonomic tone (see Table II-2-7)
λPrevent baroreceptor reflex changes in heart rate (see Figure II-2-4)
50
Chapter 2 λ Cholinergic Pharmacology
Table II-2-7. Characteristics of Ganglion Blocking Agents
Effector |
|
System |
|
Effect of Ganglion Blockade |
Arterioles |
|
SANS |
Vasodilation, hypotension |
|
Veins |
|
SANS |
Dilation, ↓ venous return, ↓ CO |
|
Heart |
|
PANS |
Tachycardia |
|
Iris |
|
PANS |
Mydriasis |
|
Ciliary muscle |
|
PANS |
Cycloplegia |
|
GI tract |
|
PANS |
↓ tone and motility—constipation |
|
Bladder |
|
PANS |
Urinary retention |
|
Salivary glands |
|
PANS |
Xerostomia |
|
Sweat glands |
|
SANS |
Anhydrosis |
|
Vasomotor |
|
Baroreceptors |
center |
|
|
|
|
CNS |
|
Blood pressure |
|
|
|
|
|
|
PANS |
change |
|
|
|
Ganglion blocking drugs |
|
|
|
|
|
|
SANS |
Heart |
prevent reflex changes |
|
in heart rate elicited |
||
|
|
Blood vessel |
by vasoconstriction (α1) |
|
|
|
and vasodilation (β2). |
Ganglionic blocking
drugs do not prevent Vasoconstrictor Vasodilator
changes in HR elicited directly by the drug (β1 or M2 agonists).
Figure II-2-4. Algorithm: Reflex Control of Heart Rate
Neuromuscular Blocking Drugs
See CNS Pharmacology, chapter on Drugs Used in Anesthesia.
51
Section II λ Autonomic Pharmacology
Chapter Summary
λAcetylcholine (ACh) is synthesized from acetate and choline in the synaptic nerve via choline acetyltransferase and is stored in the synaptic vesicles and released by Ca2+ influx upon depolarization. The ACh then binds to a receptor on the other side of the synaptic junction, thereby transmitting the signal. Acetylcholinesterase (AChE) hydrolyzes the ACh and ends the signal. Cholinergic drugs are those that affect this process either by influencing ACh levels or by acting directly on the nicotinic or muscarinic receptors.
λCholine uptake is inhibited by hemicholinium. Botulinum toxin binds to synaptobrevin and prevents ACh release, and AChE inhibitors slow its rate of breakdown. Several reversible AChE inhibitors are useful pharmacologic agents; the irreversible AChE inhibitors are generally poisons.
λA cholinomimetic is a drug that imitates ACh. Nicotine acts as a cholinomimetic on nicotinic receptors, whereas bethanechol and pilocarpine are cholinomimetics that act on muscarinic receptors.
λOther drugs are ACh receptor blockers. Specific blocking agents acting on
ganglionic nicotinic (NN) receptors are hexamethonium and mecamylamine.
Those acting on the end-plate nicotinic receptors (NM) are tubocurarine, atracurium, and succinylcholine. Those acting on muscarinic (M) receptors include atropine, benztropine, and scopolamine.
λAll M-receptor activators are nonspecific (they act on M1–3), and, in general, M-receptor activation decreases cardiovascular function and increases secretions and smooth muscle contraction. Table II-2-1 shows the type of M receptor involved and specific end-organ responses to M-receptor activators.
λTable II-2-2 summarizes the effects of nicotinic receptor activation on the adrenal medulla, the autonomic ganglia, and the neuromuscular junction.
The effect of autonomic ganglia stimulation depends upon the transmission system used to connect the ganglia to the end organ. Blood vessels are innervated by SANS, resulting in vasoconstriction. PANS innervates the gut, the end result being increased motility and secretion.
λTable II-2-3 summarizes the receptor mechanisms used by the various receptor types.
λTable II-2-4 summarizes the activity, properties, and clinical uses for the direct-acting cholinomimetics, and Table II-2-5 does the same for the indirect-acting ones.
λLong-acting AChE inhibitors are commonly used as insecticides. Although these are less toxic for humans, they still provide a hazard, causing poisoning with both acute and chronic symptoms caused by both muscarinic and nicotinic hyperactivity (“dumbbeelss”).
λTherapy for acute poisoning by AChE inhibitors includes administration of M blockers (atropine) and pralidoxime (2-PAM), which helps reactivate AChE.
52

Chapter 2 λ Cholinergic Pharmacology
Chapter Summary (cont’d )
λAtropine is the prototype of muscarinic receptor antagonist drugs. In simple terms, increasing doses of atropine progressively decreases secretions and causes mydriasis, blurred vision, tachycardia, constipation, and urinary retention. Overdoses of over-the-counter medications containing M blockers are common causes of toxicity. Management is largely symptomatic, although physostigmine may be useful because it helps counteract both central and peripheral effects. The clinical uses and properties of the M-blocking drugs are summarized in Table II-2-6.
λThe NN antagonists act as ganglionic blockers, so they will affect both the SANS and PANS tracts.
λGanglionic blockade prevents ANS reflexes. Table II-2-7 summarizes specific effects of ganglionic blocking agents and the transmission system employed for various specific organs.
λFigure II-2-4 summarizes the effects of ganglionic blockers on drugs that modify blood pressure, causing a reflex change in heart rate, and on drugs that act directly at the SA node to change the heart rate.
53

Adrenergic Pharmacology |
3 |
Learning Objectives
Answer questions about catecholamine synthesis, action, and degradation
Explain information related to direct-acting adrenoceptor agonists and indirect-acting adrenergic receptor agonists
Differentiate between alpha receptor antagonists and beta receptor antagonists
CATECHOLAMINE SYNTHESIS, ACTION, AND
DEGRADATION
The important aspects of the adrenergic neuroeffector junction are summarized in
Figure II-3-1.
55
|
Section II λ Autonomic Pharmacology |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dopa |
|
|
Tyrosine |
Tyrosine |
|||
|
|
|
|
|
|
Hydroxylase |
|||||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
Dopa |
Decarboxylase (aromatic |
|
|||||
|
|
|
|
|
amino acid decarboxylase) |
|
|||||
|
|
|
|
Dopamine |
|
|
|
||||
1 |
MAO inhibitors |
|
|
|
|
|
|||||
|
|
|
Vesicular dopamine β |
|
|
||||||
|
|
|
|
|
|
|
|||||
2 |
Releasers |
|
|
|
Hydroxylase |
|
|
||||
|
|
|
|
|
|
|
|||||
3 |
Reuptake blockers |
|
Norepinephrine |
|
|
|
|||||
4 |
α2 agonists and antagonists |
|
MAO-A |
(NE) |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
||||
5 |
Agonists and blockers of α1, |
|
NE |
|
|
|
|
|
|
|
|
|
β1 receptors |
|
Mobile Pool |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
NE |
– |
|
|
||||
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
α |
|
||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
Exocytosis |
|
|
|
|
2 |
|
||
|
|
|
|
|
|
|
receptors |
|
|||
|
|
|
Reuptake |
|
|
|
|
|
+ |
|
|
|
|
|
|
NE |
|
|
Metabolites |
|
|
||
|
|
|
+ |
COMT |
|
|
|||||
|
|
|
|
|
+ |
|
|
||||
|
|
|
|
|
|
|
|
||||
|
|
|
|
Receptors |
|
|
|
||||
|
|
|
|
Effector Cells |
|
|
|
||||

 Junction
Junction
Tyrosine is actively transported into nerve endings and is converted to dihydroxyphenylalanine (DOPA) via tyrosine hydroxylase (1). This step is rate limiting in the synthesis of NE. DOPA is converted to dopamine (DA) via L-aromatic amino acid decarboxylase (DOPA decarboxylase). DA is taken up into storage vesicles where it is metabolized to NE via DA beta hydroxylase (6). Inactivation of NE via monoamine oxidase A (MAO-A) (2) may regulate prejunctional levels of transmitter in the mobile pool (3) but not the NE stored in granules.
Presynaptic membrane depolarization opens voltage-dependent Ca2+ channels. Influx of this ion causes fusion of the synaptic granular membranes, with the presynaptic membrane leading to NE exocytosis into the neuroeffector junction
(7). NE then activates postjunctional receptors (8), leading to tissue-specific responses depending on the adrenoceptor subtype activated.
Termination of NE actions is mainly due to removal from the neuroeffector junction back into the sympathetic nerve ending via an NE reuptake transporter system
(4). At some sympathetic nerve endings, the NE released may activate prejunctional
56
Chapter 3 λ Adrenergic Pharmacology
alpha adrenoceptors (5) involved in feedback regulation, which results in decreased release of the neurotransmitter. Metabolism of NE is by catechol-O-methyltrans- ferase (COMT) in the synapse or MAOA in the prejunctional nerve terminal.
Table II-3-1. Adrenergic Receptor Activation
Receptor
α1
Eye—radial (dilator) muscle Arterioles (skin, viscera)
Veins
Bladder trigone and sphincter and prostatic urethra
Male sex organs Liver
Kidney
α2
Prejunctional nerve terminals Platelets
Pancreas
β1
Heart SA node AV node
Atrial and ventricular muscle
His-Purkinje
Kidney
β2 (mostly not innervated)
Blood vessels (all)
Uterus
Bronchioles
Skeletal muscle
Liver
Pancreas
D1 (peripheral)
Renal, mesenteric, coronary vasculature
Response
Contraction—mydriasis
Contraction—↑ TPR—↑ diastolic pressure, ↑ afterload
Contraction—↑ venous return—↑ preload Contraction—urinary retention
Vas deferens—ejaculation ↑ glycogenolysis
↓renin release
↓transmitter release and NE synthesis Aggregation
↓insulin secretion
↑HR (positive chronotropy)
↑conduction velocity (positive dromotropy)
↑force of contraction (positive inotropy), conduction velocity, CO and oxygen consumption
↑automaticity and conduction velocity
↑renin release
Vasodilation—↓ TPR—↓ diastolic pressure— ↓ afterload
Relaxation Dilation
↑glycogenolysis—contractility (tremor)
↑glycogenolysis
↑insulin secretion
Vasodilation—in kidney ↑ RBF, ↑ GFR, ↑ Na+ secretion
In A Nutshell
Adrenoceptor Sensitivity
Beta receptors are usually more sensitive to activators than alpha receptors. With drugs that exert both effects, the beta responses are dominant at low doses; at higher doses, the alpha responses will predominate.
Note
Dopamine Use in Shock
D1 |
β1 |
α1 |
increasing doses of dopamine
Fenoldopam is a D1 agonist used for severe hypertension.
57
Section II λ Autonomic Pharmacology
Table II-3-2. Mechanisms Used by Adrenergic Receptors
α1 |
Gq coupled |
↑ phospholipase C → ↑ IP3, DAG, Ca2+ |
α2 |
Gi coupled |
↓ adenylyl cyclase → ↓ cAMP |
β1 β2 D1 |
Gs coupled |
↑ adenylyl cyclase → ↑ cAMP |
|
|
|
DIRECT-ACTING ADRENOCEPTOR AGONISTS
α1 Agonists
• α1: ↑ TPR, ↑ BP
• Potential reflex bradycardia
• No change in pulse pressure
α1 activation (e.g., Phenylephrine)
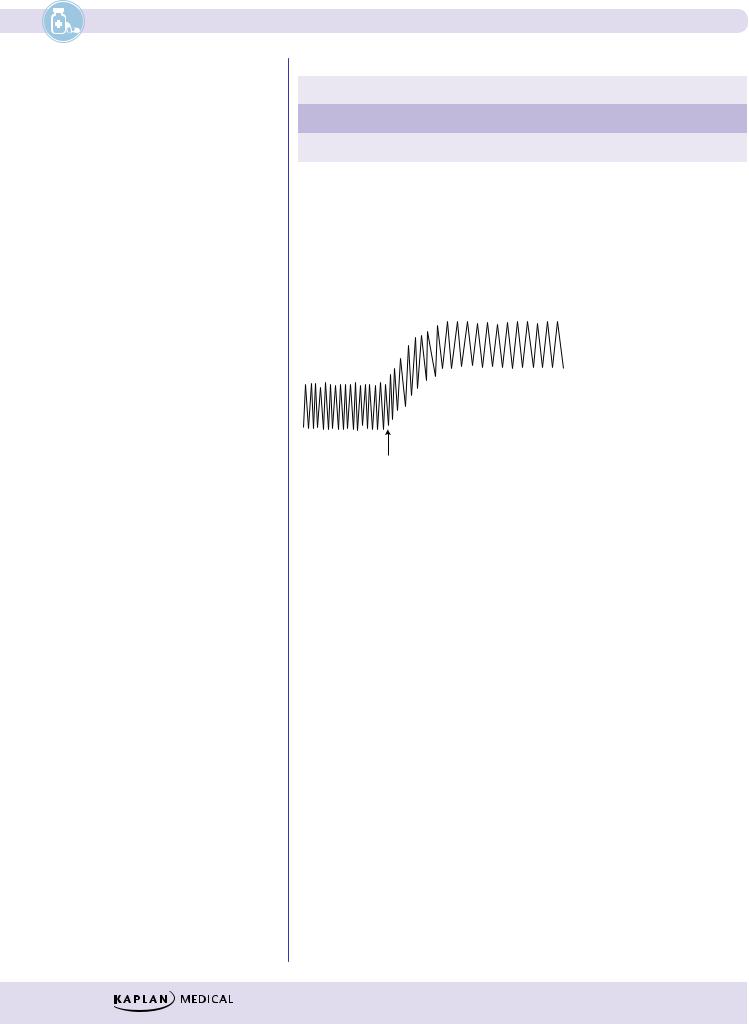
Figure II-3-2. Effect of Alpha Activators on Heart Rate and Blood Pressure
λSystemically, ↑ mean blood pressure via vasoconstriction
λ↑ BP may elicit a reflex bradycardia
λCardiac output may be ↓ but also offset by ↑ venous return
λDrugs and uses:
−Phenylephrine: nasal decongestant and ophthalmologic use (mydriasis without cycloplegia), hypotensive states
α2 Agonists
Stimulate prejunctional receptors in the CNS to decrease sympathetic outflow. Primary use is in mild to moderate HTN.
λDrugs and uses: clonidine and methyldopa (mild to moderate hypertension)
λSee Cardiovascular section.
58
