
- •Foreword
- •Preface
- •Contents
- •1. Introduction to Pathology
- •2. Techniques for the Study of Pathology
- •6. Inflammation and Healing
- •8. Neoplasia
- •16. The Heart
- •17. The Respiratory System
- •18. The Eye, ENT and Neck
- •20. The Gastrointestinal Tract
- •24. The Female Genital Tract
- •25. The Breast
- •26. The Skin
- •27. The Endocrine System
- •28. The Musculoskeletal System
- •29. Soft Tissue Tumours
- •30. The Nervous System
- •Appendix
- •Further Readings
- •Index
754
Pathology Systemic III SECTION
Chapter 25 |
The Breast |
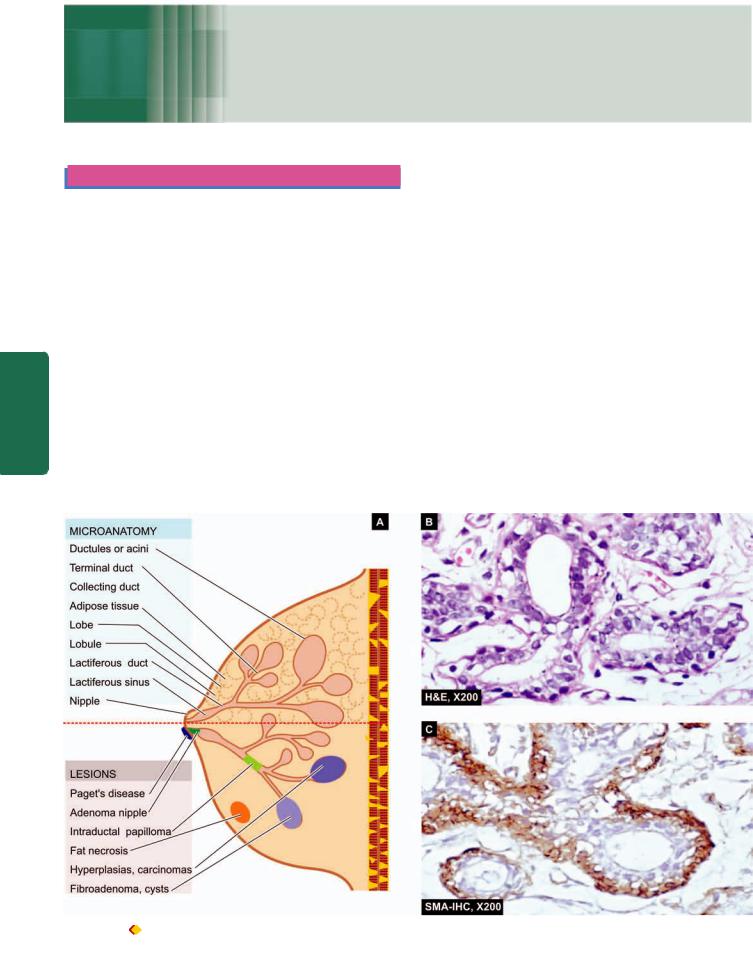
NORMAL STRUCTURE
The breast is a modified skin appendage which is functional in the females during lactation but is rudimentary in the males. Microanatomy of the breast reveals 2 types of tissue components: epithelial and stromal (Fig. 25.1). In a fullydeveloped non-lactating female breast, the epithelial component comprises less than 10% of the total volume but is more significant pathologically since majority of lesions pertain to this portion of the breast.
EPITHELIAL COMPONENT. The epithelial component of the breast consists of 2 major parts: terminal duct-lobular unit (TDLU) which performs the main secretory function during lactation, and large duct system which performs the function of collection and drainage of secretions; both are interconnected to each other.
The breast is divided into about 20 lobes. Each lobe consists of breast lobules which drain their secretions through its collecting duct system and opens into the nipple through its own main excretory duct, lactiferous duct. The segment of
lactiferous duct subjacent to the nipple shows a small dilatation called lactiferous sinus. Each lactiferous duct has its own collecting duct system which has branches of smaller diameter, ultimately terminating peripherally as terminal ducts (or TDLU) in the breast lobules.
The entire ductal-lobular epithelial system has bilayered lining: the inner epithelium with secretory and absorptive function, and an outer supporting myoepithelial lining, both having characteristic ultrastructure and immunoreativity. The inner epithelium stains positive for epithelial membrane antigen (EMA) and lactalbumin while the myoepithelium is positive for smooth muscle actin (SMA) and S-100.
STROMAL COMPONENT. The supportive stroma of the breast consists of variable amount of loose connective tissue and adipose tissue during different stages of reproductive life. The stromal tissue of the breast is present at 2 locations: intralobular and interlobular stroma. Intralobular stroma encloses each lobule, and its acini and ducts, and is chiefly made of loose connective tissue, myxomatous stroma and a few scattered lymphocytes. Interlobular stroma separates one
Figure 25.1 |
A, Microanatomy of the breast and major lesions at various sites. B, Normal terminal duct-lobular unit (TDLU). C, SMA-100 |
immunostain for myoepithelium.

lobule from the other and is composed mainly of adipose tissue and some loose connective tissue.
The most important disease of the breast is cancer. However, there are a few inflammatory lesions, benign tumours and tumour-like lesions which may be confused clinically with breast cancer. These pathologic lesions are described first, followed by an account of breast cancer.
NON-NEOPLASTIC CONDITIONS
These conditions in the breast include inflammations, fibrocystic change and gynaecomastia.
INFLAMMATIONS
Inflammation of the breast is called mastitis. Important types of mastitis are acute mastitis and breast abscess, chronic mastitis, mammary duct ectasia (or plasma cell mastitis), traumatic fat necrosis and galactocele.
Acute Mastitis and Breast Abscess
Acute pyogenic infection of the breast occurs chiefly during the first few weeks of lactation and sometimes by eczema of the nipples. Bacteria such as staphylococci and streptococci gain entry into the breast by development of cracks and fissures in the nipple. Initially a localised area of acute inflammation is produced which, if not effectively treated, may cause single or multiple breast abscesses. Extensive necrosis and replacement by fibrous scarring of the breast with retraction of the nipple may result.
Granulomatous Mastitis
Although chronic non-specific mastitis is uncommon, chronic granulomatous inflammation in the breast may occur as a result of the following:
1.Systemic non-infectious granulomatous disease e.g. as part of systemic sarcoidosis, Wegener’s granulomatosis.
2.Infections e.g. tuberculosis which is not so uncommon in developing countries like India and may be misdiagnosed clinically as breast cancer owing to axillary nodal involvement. Tubercle bacilli reach the breast by haematogenous, lymphatic or direct spread, usually from the lungs or pleura. Pathologically, typical caseating tubercles with discharging sinuses through the surface of the breast are found. ZN staining may demonstrate acid-fast bacilli. Fungal infection of the breast may occur in immunocompromised patients.
3.Silicone breast implants implanted on breast cancer patients after mastectomy or as breast augmentation cosmetic surgery may rupture or silicone may slowly leak into surrounding breast tissue. This incites chronic inflammatory reaction of lymphocytes, macrophages and foreign body giant cells. Eventually, a surrounding fibrous capsule forms and after a long period it may even be calcified.
4.Idiopathic granulomatous mastitis is an uncommon form of reaction around lobules and ducts in the absence of any known etiology. Exact pathogenesis is not known but probably it is a form of hypersensitivity reaction to luminal secretion of the breast epithelium during lactation.
Mammary Duct Ectasia (Plasma Cell Mastitis) |
755 |
Mammary duct ectasia is a condition in which one or more of the larger ducts of the breast are dilated and filled with inspissated secretions. These are associated with periductal and interstitial chronic inflammatory changes. Duct ectasia affects women in their 4th to 7th decades of life. The patients may remain asymptomatic or there may be nipple discharge, retraction of the nipple due to fibrous scarring and clinically palpable dilated ducts in the subareolar area. The lesion may be mistaken for carcinoma of the breast. The etiology of the condition remains unknown but it appears to begin with periductal inflammation followed by destruction of the elastic tissue to cause ectasia and periductal fibrosis.
MORPHOLOGIC FEATURES. Grossly, the condition |
|
|
appears as a single, poorly-defined indurated area in the |
|
|
breast with ropiness on the surface. Cut section shows |
|
|
dilated ducts containing cheesy inspissated secretions. |
|
|
Histologically, the features are as under: |
|
|
1. Dilated ducts with either necrotic or atrophic lining |
|
|
by flattened epithelium and lumen containing granular, |
|
CHAPTER |
impressive numbers and the condition is then termed |
|
|
amorphous, pink debris and foam cells. |
|
|
2. Periductal and interstitial chronic inflammation, |
|
|
chiefly lymphocytes, histiocytes with multinucleate histio- |
|
|
cytic giant cells. Sometimes, plasma cells are present in |
|
25 |
plasma cell mastitis. |
|
|
|
|
|
3. Occasionally, there may be obliteration of the ducts |
|
|
by fibrous tissue and varying amount of inflammation and |
|
The |
is termed obliterative mastitis. |
|
|
|
|
|
Fat Necrosis |
Breast |
|
|
||
Focal fat necrosis of an obese and pendulous breast followed |
|
|
by an inflammatory reaction is generally initiated by |
|
|
trauma. The condition presents as a well-defined mass with |
|
|
indurated appearance. |
|
|
|
|
|
Grossly, the excised lump has central pale cystic area of |
|
|
necrosis. |
|
|
Histologically, there is disruption of the regular pattern |
|
|
of lipocytes with formation of lipid-filled spaces |
|
|
surrounded by neutrophils, lymphocytes, plasma cells |
|
|
and histiocytes having foamy cytoplasm and frequent |
|
|
foreign body giant cell formation. In late stage, there is |
|
|
replacement fibrosis and even calcification. |
|
|
|
|
|
Galactocele |
|
|
A galactocele is cystic dilatation of one or more ducts |
|
|
occurring during lactation. The mammary duct is obstruc- |
|
|
ted and dilated to form a thin-walled cyst filled with milky |
|
|
fluid. Rarely, the wall of galactocele may get secondarily |
|
|
infected. |
|
|
FIBROCYSTIC CHANGE |
|
|
Fibrocystic change is the most common benign breast |
|
|
condition producing vague ‘lumpy’ breast rather than |
|
|
756
Pathology Systemic III SECTION
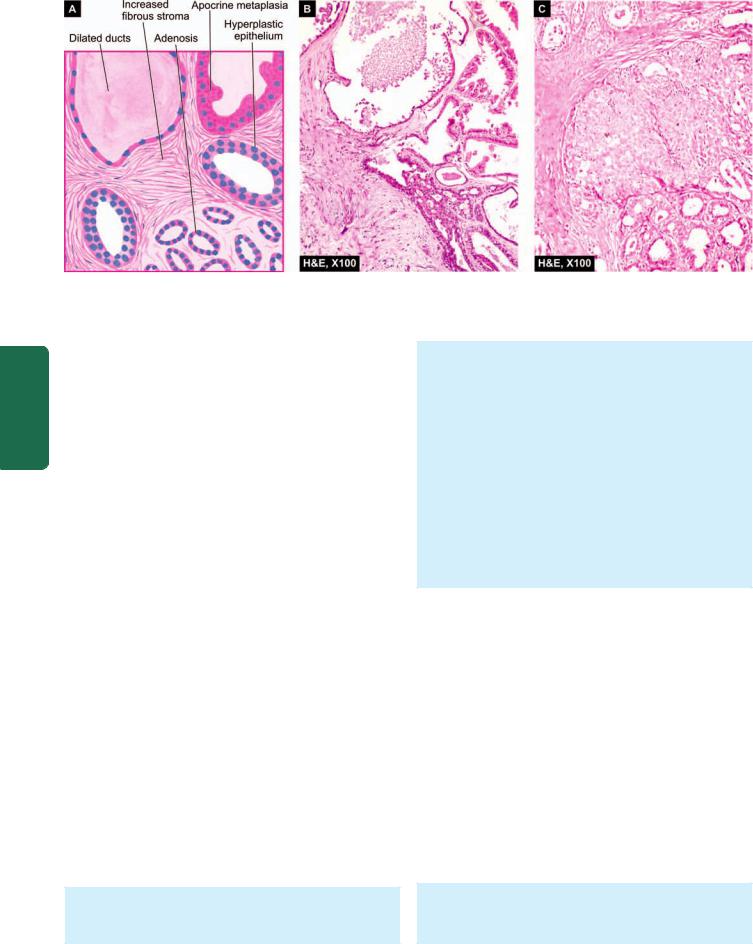
Figure 25.2 
 Simple fibrocystic change. A, Diagrammatic view. It shows cystic dilatation of ducts and increase in fibrous stroma. There is mild epithelial hyperplasia in terminal ducts. B, Non-proliferative fibrocystic changes—fibrosis, cyst formation, adenosis and apocrine metaplasia.
Simple fibrocystic change. A, Diagrammatic view. It shows cystic dilatation of ducts and increase in fibrous stroma. There is mild epithelial hyperplasia in terminal ducts. B, Non-proliferative fibrocystic changes—fibrosis, cyst formation, adenosis and apocrine metaplasia.
C, Proliferative fibrocystic changes showing moderate epithelial hyperplasia.
palpable lump in the breast. Its incidence has been reported to range from 10-20% in adult women, most often between 3rd and 5th decades of life, with dramatic decline in its incidence after menopause suggesting the role of oestrogen in its pathogenesis. It was previously termed fibrocystic disease but is currently considered as an exaggerated physiologic phenomena and not a disease. The entity was formerly also known as benign mammary dysplasia under the mistaken belief that all forms are dysplastic or precancerous conditions, and hence has attracted considerable interest.
As such, fibrocystic change of the female breast is a histologic entity characterised by following features:
i)Cystic dilatation of terminal ducts.
ii)Relative increase in interand intralobular fibrous tissue.
iii)Variable degree of epithelial proliferation in the terminal ducts.
It is important to identify the spectrum of histologic features by core needle biopsy or cytologic findings by FNAC in fibrocystic changes since only some subset of changes has an increased risk of development of breast cancer. Presently, the spectrum of histologic changes are divided into two clinicopathologically relevant groups:
A. Nonproliferative changes: Simple fibrocystic change. B. Proliferative changes: Proliferative fibrocystic change.
A.Nonproliferative Fibrocystic Changes: Simple Fibrocystic Change
Simple fibrocystic change most commonly includes 2 features—formation of cysts of varying size, and increase in fibrous stroma. Cysts are formed by dilatation of obstructed collecting ducts, obstruction being caused by periductal fibrosis following inflammation or fibrous overgrowth from oestrogen stimulation.
MORPHOLOGIC FEATURES. Grossly, the cysts are rarely solitary but are usually multifocal and bilateral. They vary from microcysts to 5-6 cm in diameter. The
usual large cyst is rounded, translucent with bluish colour prior to opening (blue-dome cyst). On opening, the cyst contains thin serous to haemorrhagic fluid.
Microscopically, simple fibrocystic change includes following 2 features (Fig. 25.2):
1.Cyst formation: The cyst lining shows a variety of appearances. Often, the epithelium is flattened or atrophic. Frequently, there is apocrine change or apocrine metaplasia in the lining of the cyst resembling the cells of apocrine sweat glands. Occasionally, there is simultaneous epithelial hyperplasia (discussed below) forming tiny intracystic papillary projections of piled up epithelium.
2.Fibrosis: There is increased fibrous stroma surrounding the cysts and variable degree of stromal lymphocytic infiltrate.
B.Proliferative Fibrocystic Changes;
Epithelial Hyperplasia and Sclerosing Adenosis
Proliferative fibrocystic change in the breasts includes 2 entities: epithelial hyperplasia and sclerosing adenosis.
EPITHELIAL HYPERPLASIA. Epithelial hyperplasia (or epitheliosis in the British literature) is defined as increase in the layers of epithelial cells over the basement membrane to three or more layers in the ducts (ductal hyperplasia) or lobules (lobular hyperplasia). The latter condition, lobular hyperplasia, must be distinguished from adenosis (discussed separately) in which there is increase in the number of ductules or acini without any change in the number or type of cells lining them. Epithelial hyperplasia may be totally benign or may have atypical features. It is the latter type of hyperplasia which is precancerous and is associated with increased risk of developing breast cancer.
Microscopically, epithelial hyperplasia is characterised by epithelial proliferation to more than its normal double layer. In general, ductal hyperplasia is termed as epithelial
hyperplasia of usual type and may show various grades of epithelial proliferations (mild, moderate and atypical) as under, while lobular hyperplasia involving the ductules or acini is always atypical:
1.Mild hyperplasia of ductal epithelium consists of at least three layers of cells above the basement membrane, present focally or evenly throughout the duct.
2.Moderate and florid hyperplasia of ductal type is associated with tendency to fill the ductal lumen with proliferated epithelium. Such epithelial proliferations into the lumina of ducts may be focal, forming papillary epithelial projections called ductal papillomatosis, or may be more extensive, termed florid papillomatosis, or may fill the ductal lumen leaving only small fenestrations in it.
3.Of all the ductal hyperplasias, atypical ductal hyperplasia is more ominous and has to be distinguished from intraductal carcinoma (page 760). The proliferated epithelial cells in the atypical ductal hyperplasia partially fill the duct lumen and produce irregular microglandular spaces or cribriform pattern. The individual cells are uniform in shape but show loss of polarity with indistinct cytoplasmic margin and slightly elongated nuclei.
4.Atypical lobular hyperplasia is closely related to lobular carcinoma in situ (page 761) but differs from the latter in having cytologically atypical cells only in half of the ductules or acini.
SCLEROSING ADENOSIS. Sclerosing adenosis is benign proliferation of small ductules or acini and intralobular fibrosis. The lesion may be present as diffusely scattered microscopic foci in the breast parenchyma, or may form an isolated palpable mass which may simulate an infiltrating carcinoma, both clinically and pathologically.
Grossly, the lesion may be coexistent with other components of fibrocystic disease, or may form an isolated mass which has hard cartilage-like consistency, resembling an infiltrating carcinoma.
Microscopically, there is proliferation of ductules or acini and fibrous stromal overgrowth. The histologic appearance may superficially resemble infiltrating carcinoma but differs from the latter in having maintained lobular pattern and lack of infiltration into the surrounding fat.
Prognostic Significance
Since there is a variable degree of involvement of epithelial and mesenchymal elements in fibrocystic change, following prognostic implications may occur:
1.Simple fibrocystic change or nonproliferative fibrocystic changes of fibrosis and cyst formation do not carry any increased risk of developing invasive breast cancer.
2.Identification of general proliferative fibrocystic changes are associated with 1.5 to 2 times increased risk for development of invasive breast cancer.
3.Multifocal and bilateral proliferative changes in the breast pose increased risk to both the breasts equally.
4.Within the group of proliferative fibrocystic changes, atypical hyperplasia in particular, carries 4 to 5 times increased
risk to develop invasive breast cancer later. This risk is further more if there is a history of breast cancer in the family.
GYNAECOMASTIA (HYPERTROPHY OF MALE BREAST)
Unilateral or bilateral enlargement of the male breast is known as gynaecomastia. Since the male breast does not contain secretory lobules, the enlargement is mainly due to proliferation of ducts and increased periductal stroma. Gynaecomastia occurs in response to hormonal stimulation, mainly oestrogen. Such excessive oestrogenic activity in males is seen in young boys between 13 and 17 years of age
(pubertal gynaecomastia), in men over 50 years (senescent gynaecomastia), in endocrine diseases associated with increased oestrogenic or decreased androgenic activity e.g. in hepatic cirrhosis, testicular tumours, pituitary tumours, carcinoma of the lung, exogenous oestrogen therapy as in carcinoma of the prostate and testicular atrophy in Klinefelter’s syndrome (secondary gynaecomastia); and lastly, enlargement without any obvious cause (idiopathic gynaeco-
mastia).
MORPHOLOGIC FEATURES. Grossly, one or both the male breasts are enlarged having smooth glistening white tissue.
Microscopically, there are 2 main features:
1.Proliferation of branching ducts which display epithelial hyperplasia with formation of papillary projections at places.
2.Increased fibrous stroma with, myxoid appearance.
BREAST TUMOURS
Tumours of the female breast are common and clinically significant but are rare in men. Among the important benign breast tumours are fibroadenoma, phyllodes tumour (cystosarcoma phyllodes) and intraductal papilloma. Carcinoma of the breast is an important malignant tumour which occurs as non-invasive (carcinoma in situ) and invasive cancer with its various morphologic varieties.
FIBROADENOMA
Fibroadenoma or adenofibroma is a benign tumour of fibrous and epithelial elements. It is the most common benign tumour of the female breast. Though it can occur at any age during reproductive life, most patients are between 15 to 30 years of age. Clinically, fibroadenoma generally appears as a solitary, discrete, freely mobile nodule within the breast. Rarely, fibroadenoma may contain in situ or invasive lobular or ductal carcinoma, or the carcinoma may invade the fibroadenoma from the adjacent primary breast cancer.
MORPHOLOGIC FEATURES. Grossly, typical fibroadenoma is a small (2-4 cm diameter), solitary, wellencapsulated, spherical or discoid mass. The cut surface is firm, grey-white, slightly myxoid and may show slitlike spaces formed by compressed ducts. Occasionally, multiple fibroadenomas may form part of fibrocystic disease and is termed fibroadenomatosis. Less commonly,
757
Breast The 25 CHAPTER
758
Pathology Systemic III SECTION
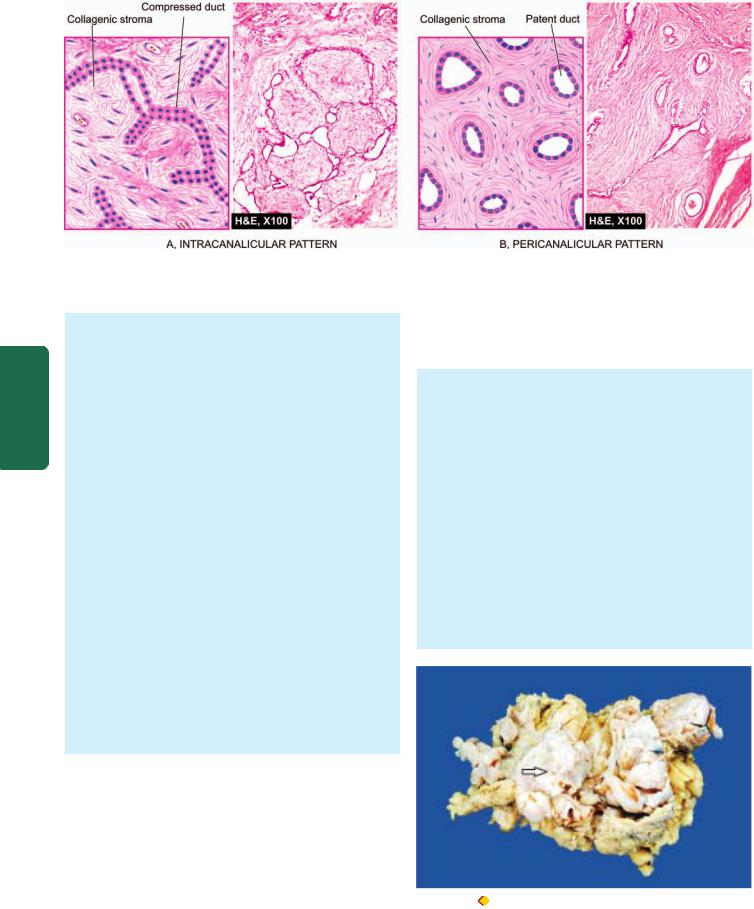
Figure 25.3 
 Fibroadenoma of the breast, microscopic patterns.
Fibroadenoma of the breast, microscopic patterns.
a fibroadenoma may be fairly large in size, up to 15 cm in diameter, and is called giant fibroadenoma but lacks the histologic features of cystosarcoma phyllodes (see below). Microscopically, fibrous tissue comprises most of a fibroadenoma. The arrangements between fibrous overgrowth and ducts may produce two types of patterns which may coexist in the same tumour. These are intracanalicular and pericanalicular patterns (Fig. 25.3):
 Intracanalicular pattern is one in which the stroma compresses the ducts so that they are reduced to slit-like clefts lined by ductal epithelium or may appear as cords of epithelial elements surrounding masses of fibrous stroma.
Intracanalicular pattern is one in which the stroma compresses the ducts so that they are reduced to slit-like clefts lined by ductal epithelium or may appear as cords of epithelial elements surrounding masses of fibrous stroma.
 Pericanalicular pattern is characterised by encircling masses of fibrous stroma around the patent or dilated ducts.
Pericanalicular pattern is characterised by encircling masses of fibrous stroma around the patent or dilated ducts.
The fibrous stroma may be quite cellular, or there may be areas of hyalinised collagen. Sometimes, the stroma is loose and myxomatous. Occasionally, the fibrous tissue element in the tumour is scanty, and the tumour is instead predominantly composed of closely-packed ductular or acinar proliferation and is termed tubular adenoma. If an adenoma is composed of acini with secretory activity, it is called lactating adenoma seen during pregnancy or lactation. Juvenile fibroadenoma is an uncommon variant of fibroadenoma which is larger and rapidly growing mass seen in adolescent girls but fortunately does not recur after excision.
PHYLLODES TUMOUR (CYSTOSARCOMA PHYLLODES)
Cystosarcoma phyllodes was the nomenclature given by Müller in 1838 to an uncommon bulky breast tumour with leaf-like gross appearance (phyllodes=leaf-like) having an aggressive clinical behaviour. Most patients are between 30 to 70 years of age. Grossly, the tumour resembles a giant fibroadenoma but is distinguished histologically from the latter by more cellular connective tissue. Later, the WHO classification of breast tumours has proposed the term ‘phyllodes tumour’ in place of misleading term of ‘cystosarcoma phyllodes’. Phyllodes tumour can be classified into
benign, borderline and malignant on the basis of histologic features. Local recurrences are much more frequent than metastases.
MORPHOLOGIC FEATURES. Grossly, the tumour is generally large, 10-15 cm in diameter, round to oval, bosselated, and less fully encapsulated than a fibroadenoma. The cut surface is grey-white with cystic cavities, areas of haemorrhages, necrosis and degenerative changes (Fig. 25.4).
Histologically, the phyllodes tumour is composed of an extremely hypercellular stroma, accompanied by proliferation of benign ductal structures. Thus, phyllodes tumour resembles fibroadenoma except for enhanced stromal cellularity. The histologic criteria used to distinguish benign, borderline and malignant categories of phyllodes tumour are as under:
 frequency of mitoses;
frequency of mitoses;
 cellular atypia;
cellular atypia;
 cellularity; and
cellularity; and  infiltrative margins.
infiltrative margins.
Figure 25.4 |
Phyllodes tumour. Simple mastectomy specimen shows |
replacement of almost whole breast with a large circumscribed, greywhite, firm, nodular mass having slit-like, compressed cystic areas (arrow) and areas of haemorrhage.

About 20% of phyllodes tumours are histologically malignant and less than half of them may metastasise.
INTRADUCTAL PAPILLOMA
Intraductal papilloma is a benign papillary tumour occurring most commonly in a lactiferous duct or lactiferous sinus near the nipple. Clinically, it produces serous or serosanguineous nipple discharge. It is most common in 3rd and 4th decades of life.
MORPHOLOGIC FEATURES. Grossly, intraductal papilloma is usually solitary, small, less than 1 cm in diameter, commonly located in the major mammary ducts close to the nipple. Less commonly, there are multiple papillomatosis which are more frequently related to a papillary carcinoma.
Histologically, an intraductal papilloma is characterised by multiple papillae having well-developed fibrovascular stalks attached to the ductal wall and covered by benign cuboidal epithelial cells supported by myoepithelial cells. An intraductal papillary carcinoma is distinguished from intraductal papilloma in having severe cytologic atypia, pleomorphism, absence of myoepithelial cells, multilayering and presence of mitotic figures.
CARCINOMA OF THE BREAST
Cancer of the breast is among the commonest of human cancers throughout the world. Its incidence varies in different countries but is particularly high in developed countries. In the United States, carcinoma of the breast constitutes about 25% of all cancers in females and causes approximately 20% of cancer deaths among females. However, there has been some decline in mortality from the breast cancer in recent years in North America, Western Europe and Australia due to both early diagnosis and modern therapy. Cancer of the male breast, on the other hand, is quite rare and comprises 0.2% of malignant tumours (ratio between male-female breast cancer is 1:100). The incidence of breast cancer is highest in the perimenopausal age group and is uncommon before the age of 25 years.
Clinically, the breast cancer usually presents as a solitary, painless, palpable lump which is detected quite often by selfexamination. Higher the age, more are the chances of breast lump turning out to be malignant. Thus, all breast lumps, irrespective of the age of the patient must be removed surgically. Currently, emphasis is on early diagnosis by mammography, xero-radiography and thermography. Techniques like fine needle aspiration cytology (FNAC), stereotactic biopsy and frozen section are immensely valuable to the surgeon for immediate pathological diagnosis.
Etiology
Though extensive clinical and experimental research as well as epidemiologic studies have been carried out in the field of breast cancer, its exact etiology remains elusive. However, based on current status of our knowledge, the following risk factors are considered significant in its etiology:
1.Geography. The incidence of breast cancer is about six times higher in developed countries than the developing countries, with the notable exception of Japan. These geographic differences are considered to be related to consumption of large amount of animal fats and high caloric diet by Western populations than the Asians (including Japanese) and Africans.
2.Genetic factors. Recently, much work has been done on the influence of family history and inherited mutations in breast cancer:
i)Family history: First-degree relatives (mother, sister, daughter) of women with breast cancer have 2 to 6-fold higher risk of development of breast cancer. The risk is proportionate to a few factors:

 Number of blood relatives with breast cancer.
Number of blood relatives with breast cancer.

 Younger age at the time of development of breast cancer.
Younger age at the time of development of breast cancer.

 Bilateral cancers.
Bilateral cancers.

 High risk cancer families having breast and ovarian carcinomas.
High risk cancer families having breast and ovarian carcinomas.
ii)Genetic mutations: About 10% breast cancers have been found to have inherited mutations. These mutations include the following, most important of which is breast cancer (BRCA) susceptibility gene in inherited breast cancer:
 BRCA 1 gene located on chromosome 17, a DNA repair gene, is implicated in both breast and ovarian cancer in inherited cases. BRCA1 deletion is seen in about two-third of women with inherited breast cancer having family history but BRCA1 mutation is uncommon in sporadic cases. The protein product of BRCA gene is a cell cycle regulated protein and it can be detected by immunohistochemistry. Men who have mutated BRCA1 have increased risk of developing cancer of the prostate but not of male breast.
BRCA 1 gene located on chromosome 17, a DNA repair gene, is implicated in both breast and ovarian cancer in inherited cases. BRCA1 deletion is seen in about two-third of women with inherited breast cancer having family history but BRCA1 mutation is uncommon in sporadic cases. The protein product of BRCA gene is a cell cycle regulated protein and it can be detected by immunohistochemistry. Men who have mutated BRCA1 have increased risk of developing cancer of the prostate but not of male breast.

 BRCA 2 gene located on chromosome 13, another DNA repair gene, in its mutated form, has a similarly higher incidence of inherited cancer of the breast (one-third cases) and ovary in females, and prostate in men.
BRCA 2 gene located on chromosome 13, another DNA repair gene, in its mutated form, has a similarly higher incidence of inherited cancer of the breast (one-third cases) and ovary in females, and prostate in men.
 In both BRCA1 and BRCA2, both copies of the genes (homozygous state) must be inactivated for development of breast cancer.
In both BRCA1 and BRCA2, both copies of the genes (homozygous state) must be inactivated for development of breast cancer.

 Mutation in p53 tumour suppressor gene on chromosome 17 as an acquired defect accounts for 40% cases of sporadic breast cancer in women but rarely in women with family history of breast cancer. p53 mutation is also seen in LiFraumeni syndrome having multiple cancers including breast cancer in young women; others are tumours of the brain, sarcomas, and adrenal cortical tumours.
Mutation in p53 tumour suppressor gene on chromosome 17 as an acquired defect accounts for 40% cases of sporadic breast cancer in women but rarely in women with family history of breast cancer. p53 mutation is also seen in LiFraumeni syndrome having multiple cancers including breast cancer in young women; others are tumours of the brain, sarcomas, and adrenal cortical tumours.
 Other mutations seen less frequently in breast cancer include ataxia telangiectasia gene, PTEN (phosphate and tensin) tumour suppressor gene.
Other mutations seen less frequently in breast cancer include ataxia telangiectasia gene, PTEN (phosphate and tensin) tumour suppressor gene.
3. Oestrogen excess. There is sufficient evidence to suggest that excess endogenous oestrogen or exogenously administered oestrogen for prolonged duration is an important factor in the development of breast cancer. Evidences in support of increased risk with oestrogen excess are as follows:
i)Women with prolonged reproductive life, with menarche setting in at an early age and menopause relatively late have greater risk.
ii)Higher risk in unmarried and nulliparous women than in married and multiparous women.
759
Breast The 25 CHAPTER

760 iii) Women with first childbirth at a late age (over 30 years) are at greater risk.
|
iv) Lactation is said to reduce the risk of breast cancer. |
|
|
v) Bilateral oophorectomy reduces the risk of development |
|
|
of breast cancer. |
|
|
vi) Functioning ovarian tumours (e.g. granulosa cell tumour) |
|
|
which elaborate oestrogen are associated with increased |
|
|
incidence of breast cancer. |
|
|
vii) Oestrogen replacement therapy administered to post- |
|
|
menopausal women may result in increased risk of breast |
|
|
cancer. |
|
|
viii) Long-term use of oral contraceptives has been suspected |
|
|
to predispose to breast cancer but there is no definite |
|
|
increased risk with balanced oestrogen-progesterone |
|
|
preparations used in oral contraceptives. |
|
|
ix) Men who have been treated with oestrogen for prostatic |
|
|
cancer have increased risk of developing cancer of the male |
|
|
breast. |
|
|
Normal breast epithelium possesses oestrogen and |
|
|
progesterone receptors. The breast cancer cells secrete many |
|
|
growth factors which are oestrogen-dependent. In this way, |
|
SECTION |
the interplay of high circulating levels of oestrogen, oestrogen |
|
receptors and growth factors brings about progression of |
||
|
||
|
breast cancer. |
|
|
4. Miscellaneous factors. These include a host of following |
|
|
environmental influences and dietary factors associated with |
|
III |
increased risk of breast cancer: |
|
i) Consumption of large amounts of animal fats, high calorie |
||
|
||
|
foods. |
|
Systemic |
ii) Cigarette smoking. |
|
iii) Alcohol consumption. |
||
|
||
|
iv) Breast augmentation surgery. |
|
|
v) Exposure to ionising radiation during breast |
|
Pathology |
developement. |
|
vi) Identification of a transmissible retrovirus in early 20th |
||
|
||
|
century, mouse mammary tumour virus (MMTV), also called |
|
|
Bittner milk factor transmitted from the infected mother-mice |
|
|
to the breast-fed daughter-mice prompted researchers to look |
|
|
for similar agent in human breast cancer (Chapter 8). Though |
|
|
no such agent has yet been identified, there are reports of |
|
|
presence of reverse transcriptase in breast cancer cells. |
|
|
5. Fibrocystic change. Fibrocystic change, particularly |
|
|
when associated with atypical epithelial hyperplasia, has |
|
|
about 5-fold higher risk of developing breast cancer |
|
|
subsequently. |
|
|
General Features and Classification |
|
|
Cancer of the breast occurs more often in left breast than |
|
|
the right and is bilateral in about 4% cases. Anatomically, |
|
|
upper outer quadrant is the site of tumour in half the breast |
|
|
cancers; followed in frequency by central portion, and |
|
|
equally in the remaining both lower and the upper inner |
|
|
quadrant as shown in Fig. 25.5. |
|
|
Carcinoma of the breast arises from the ductal epithelium |
|
|
in 90% cases while the remaining 10% originate from the |
|
|
lobular epithelium. For variable period of time, the tumour |
|
|
cells remain confined within the ducts or lobules (non- |
Figure 25.5 
 Topographic considerations in breast cancer.
Topographic considerations in breast cancer.
invasive carcinoma) before they invade the breast stroma (invasive carcinoma). While only 2 types of non-invasive carcinoma have been described—intraductal carcinoma and lobular carcinoma in situ, there is a great variety of histological patterns of invasive carcinoma breast which have clinical correlations and prognostic implications. Table 25.1 presents different types of carcinoma of the breast as proposed in the WHO classification with some modifications. The important morphological forms are described below.
A. NON-INVASIVE (IN SITU) BREAST CARCINOMA
In general, two types of non-invasive or in situ carcinoma— intraductal carcinoma and lobular carcinoma in situ, are characterised histologically by presence of tumour cells within the ducts or lobules respectively without evidence of invasion.
Intraductal Carcinoma
Carcinoma in situ confined within the larger mammary ducts is called intraductal carcinoma. The tumour initially begins with atypical hyperplasia of ductal epithelium followed by filling of the duct with tumour cells. Clinically, it produces a palpable mass in 30-75% of cases and presence of nipple discharge in about 30% patients. Approximately a quarter
TABLE 25.1: Classification of Carcinoma of the Breast.
A.NON-INVASIVE (IN SITU) CARCINOMA
1.Intraductal carcinoma
2.Lobular carcinoma in situ
B.INVASIVE CARCINOMA
1.Infiltrating (invasive) duct carcinoma-NOS (not otherwise specified)
2.Infiltrating (invasive) lobular carcinoma
3.Medullary carcinoma
4.Colloid (mucinous) carcinoma
5.Papillary carcinoma
6.Tubular carcinoma
7.Adenoid cystic (invasive cribriform) carcinoma
8.Secretory (juvenile) carcinoma
9.Inflammatory carcinoma
10.Carcinoma with metaplasia
C.PAGET’S DISEASE OF THE NIPPLE
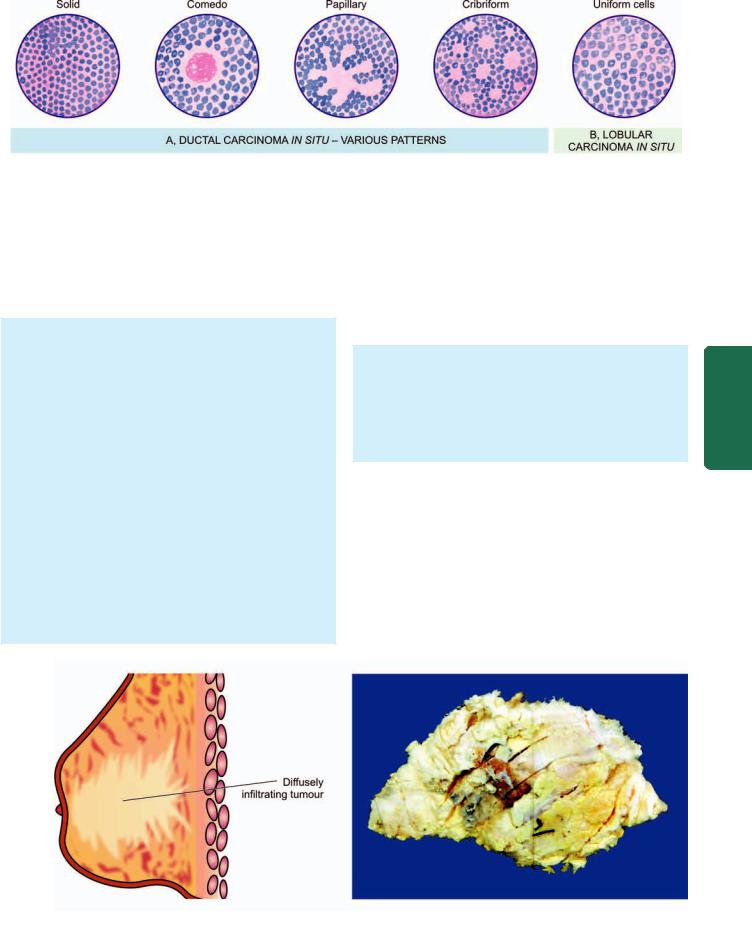
761
Figure 25.6 
 Morphologic patterns in non-invasive (in situ) carcinoma of breast.
Morphologic patterns in non-invasive (in situ) carcinoma of breast.
of patients of intraductal carcinoma treated with excisional biopsy alone develop ipsilateral invasive carcinoma during a follow-up period of 10 years while the chance of a contralateral breast cancer developing in patients with intraductal carcinoma is far less than that associated with in situ lobular carcinoma.
MORPHOLOGIC FEATURES. Grossly, the tumour may vary from a small poorly-defined focus to 3-5 cm diameter mass. On cut section, the involved area shows cystically dilated ducts containing cheesy necrotic material (in comedo pattern), or the intraductal tumour may be polypoid and friable resembling intraductal papilloma (in papillary
pattern).
Histologically, the proliferating tumour cells within the ductal lumina may have 4 types of patterns in different combinations: solid, comedo, papillary and cribriform
(Fig. 25.6,A):
i)Solid pattern is characterised by filling and plugging of the ductal lumina with tumour cells.
ii)Comedo pattern is centrally placed necrotic debris surrounded by neoplastic cells in the duct.
iii)Papillary pattern has formation of intraductal papillary projections of tumour cells which lack a fibrovascular stalk so as to distinguish it from intraductal papilloma.
iv)Cribriform pattern is recognised by neat punched out fenestrations in the intraductal tumour.
Lobular Carcinoma in Situ
Lobular carcinoma in situ is not a palpable or grossly visible tumour. Patients of in situ lobular carcinoma treated with excisional biopsy alone develop invasive cancer of the ipsilateral breast in about 25% cases in 10 years as in intraductal carcinoma but, in addition, have a much higher incidence of developing a contralateral breast cancer (30%).
MORPHOLOGIC FEATURES. Grossly, no visible tumour is identified.
Histologically, in situ lobular carcinoma is characterised by filling up of terminal ducts and ductules or acini by rather uniform cells which are loosely cohesive and have small, rounded nuclei with indistinct cytoplasmic margins
(Fig. 25.6,B).
B. INVASIVE BREAST CARCINOMA
Infiltrating (Invasive) Duct Carcinoma-NOS
Infiltrating duct carcinoma-NOS (not otherwise specified) is the classic breast cancer and is the most common histologic pattern accounting for 70% cases of breast cancer. In fact, this is the pattern of cancer for which the terms ‘cancer’ and ‘carcinoma’ were first coined by Hippocrates. Clinically, majority of infiltrating duct carcinomas have a hard consistency due to dense collagenous stroma (scirrhous
Breast The 25 CHAPTER
Figure 25.7 
 Infiltrating duct carcinoma-NOS. The breast shows a tumour extending up to nipple and areola. Cut surface shows a grey white firm tumour extending irregularly into adjacent breast parenchyma.
Infiltrating duct carcinoma-NOS. The breast shows a tumour extending up to nipple and areola. Cut surface shows a grey white firm tumour extending irregularly into adjacent breast parenchyma.
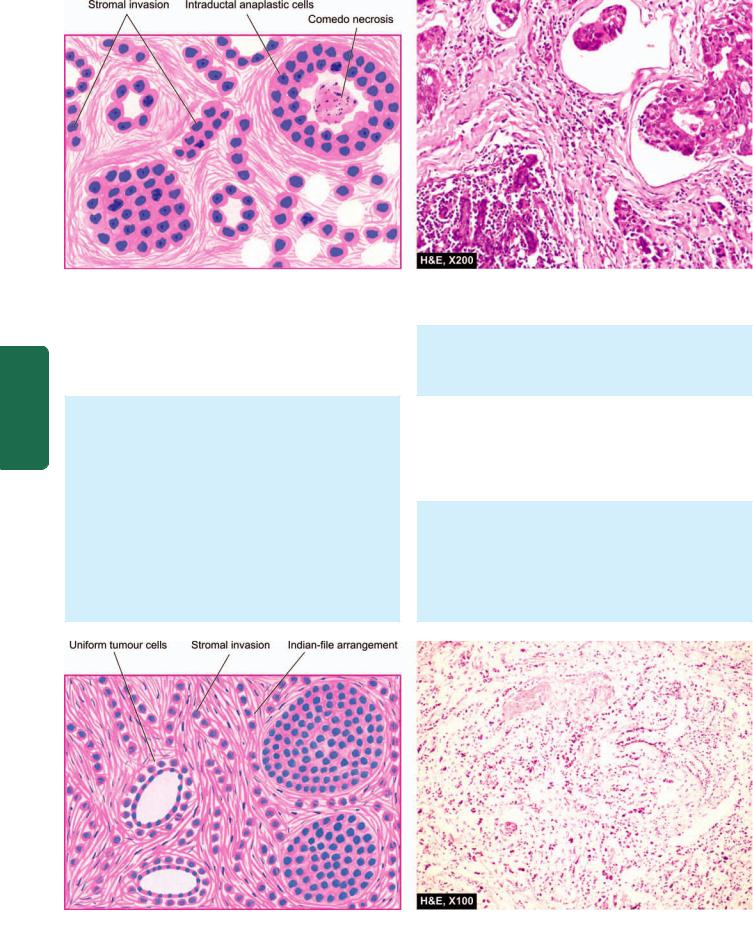
762
Pathology Systemic III SECTION
Figure 25.8 
 Infiltrating duct carcinoma-NOS. Microscopic features include formation of solid nests, cords, gland-like structures and intraductal growth pattern of anaplastic tumour cells. There is infiltration of densely collagenised stroma by these cells in a haphazard manner.
Infiltrating duct carcinoma-NOS. Microscopic features include formation of solid nests, cords, gland-like structures and intraductal growth pattern of anaplastic tumour cells. There is infiltration of densely collagenised stroma by these cells in a haphazard manner.
carcinoma). They are found more frequently in the left breast, often in the upper outer quadrant. Retraction of the nipple and attachment of the tumour to underlying chest wall may be present.
MORPHOLOGIC FEATURES. Grossly, the tumour is irregular, 1-5 cm in diameter, hard cartilage-like mass that cuts with a grating sound. The sectioned surface of the tumour is grey-white to yellowish with chalky streaks and often extends irregularly into the surrounding fat
(Fig. 25.7).
Histologically, as the name NOS suggests, the tumour is different from other special types in lacking a regular and uniform pattern throughout the lesion. A variety of histologic features commonly present are as under
(Fig. 25.8):
i) Anaplastic tumour cells forming solid nests, cords, poorly-formed glandular structures and some intraductal foci.
ii)Infiltration by these patterns of tumour cells into diffuse fibrous stroma and fat.
iii)Invasion into perivascular and perineural spaces as well as lymphatic and vascular invasion.
Infiltrating (Invasive) Lobular Carcinoma
Invasive lobular carcinoma comprises about 5% of all breast cancers. This peculiar morphologic form differs from other invasive cancers in being more frequently bilateral; and within the same breast, it may have multicentric origin.
MORPHOLOGIC FEATURES. Grossly, the appearance varies from a well-defined scirrhous mass to a poorlydefined area of induration that may remain undetected by inspection as well as palpation.
Histologically, there are 2 distinct features (Fig. 25.9):
i) Pattern—A characteristic single file (Indian file) linear arrangement of stromal infiltration by the tumour cells
Figure 25.9 
 Invasive lobular carcinoma. Characteristic histologic features are: one cell wide files of round regular tumour cells (‘Indian file’ arrangement) infiltrating the stroma and arranged circumferentially around ducts in a target-like pattern.
Invasive lobular carcinoma. Characteristic histologic features are: one cell wide files of round regular tumour cells (‘Indian file’ arrangement) infiltrating the stroma and arranged circumferentially around ducts in a target-like pattern.
with very little tendency to gland formation is seen. Infiltrating cells may be arranged concentrically around ducts in a target-like pattern.
ii) Tumour cytology—Individual tumour cells resemble cells of in situ lobular carcinoma. They are round and regular with very little pleomorphism and infrequent mitoses. Some tumours may show signet-ring cells distended with cytoplasmic mucin.
Medullary Carcinoma
Medullary carcinoma is a variant of ductal carcinoma and comprises about 1% of all breast cancers. The tumour has a significantly better prognosis than the usual infiltrating duct carcinoma, probably due to good host immune response in the form of lymphoid infiltrate in the tumour stroma.
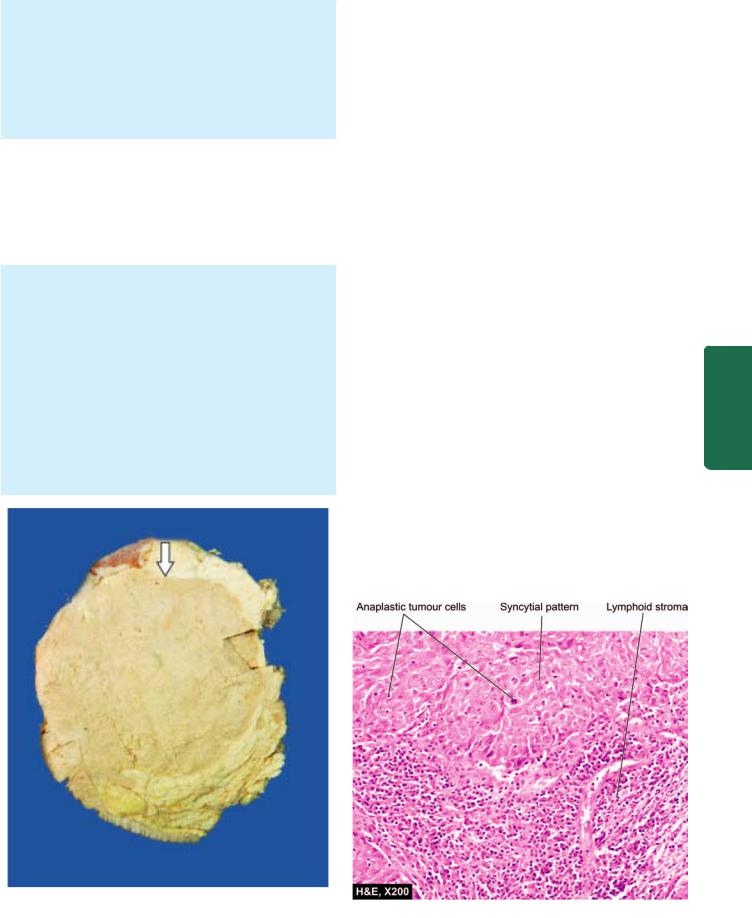
MORPHOLOGIC FEATURES. Grossly, the tumour is characterised by a large, well-circumscribed, rounded mass that is typically soft and fleshy or brain-like and hence the alternative name of ‘encephaloid carcinoma’. Cut section shows areas of haemorrhages and necrosis
(Fig. 25.10).
Histologically, medullary carcinoma is characterised by
2distinct features (Fig. 25.11):
i)Tumour cells—Sheets of large, pleomorphic tumour cells with abundant cytoplasm, large vesicular nuclei and many bizarre and atypical mitoses are diffusely spread in the scanty stroma.
ii)Stroma—The loose connective tissue stroma is scanty and usually has a prominent lymphoid infiltrate.
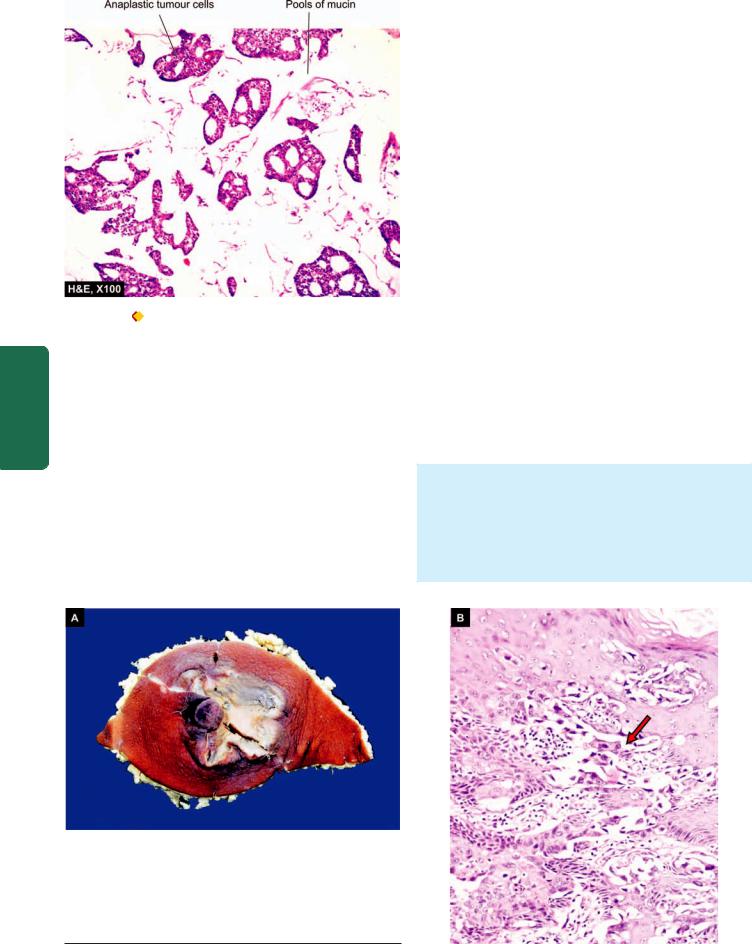
Colloid (Mucinous) Carcinoma |
763 |
||
This is an uncommon pattern of breast cancer occurring more |
|
||
frequently in older women and is slow-growing. Colloid |
|
||
carcinoma has better prognosis than the usual infiltrating |
|
||
duct carcinoma. |
|
||
|
|
||
MORPHOLOGIC FEATURES. Grossly, the tumour is |
|
|
|
usually a soft and gelatinous mass with well-demarcated |
|
|
|
borders. |
|
|
|
Histologically, colloid carcinoma contains large amount |
|
|
|
of extracellular epithelial mucin and acini filled with |
|
|
|
mucin. Cuboidal to tall columnar tumour cells, some |
|
|
|
showing mucus vacuolation, are seen floating in large |
|
|
|
lakes of mucin (Fig. 25.12). |
|
|
|
Other Morphologic Forms |
|
||
A few other morphologic forms of invasive breast carcinoma |
|
||
having clinical significance have been recognised: |
|
||
PAPILLARY CARCINOMA. It is a rare variety of infiltrating |
|
||
duct carcinoma in which the stromal invasion is in the form |
|
||
of papillary structures. |
|
||
1. TUBULAR CARCINOMA. Tubular carcinoma is an |
CHAPTER |
||
and has an orderly pattern. The tumour cells are regular |
|||
uncommon variant of invasive ductal carcinoma which has |
|
||
more favourable prognosis. |
|
||
Histologically, the tumour is highly well-differentiated |
|
|
|
and form a single layer in well-defined tubules. The |
|
25 |
|
tubules are quite even and distributed in dense fibrous |
|
||
|
|
||
stroma. |
|
The |
|
2. ADENOID CYSTIC CARCINOMA. Adenoid cystic or |
|||
|
|||
invasive cribriform carcinoma is a unique histologic pattern |
Breast |
||
of breast cancer with excellent prognosis. |
|||
|
|
||
Histologically, there is stromal invasion by islands of cells |
|
||
having characteristic cribriform (fenestrated) appearance. |
|
|
|
|
|
|
|
Figure 25.10 
 Medullary carcinoma breast. Cut surface of the breast shows a large grey white soft fleshy tumour replacing almost whole of the breast. The tumour is somewhat delineated from the adjacent breast parenchyma (arrow) as compared to irregular margin of IDC.
Medullary carcinoma breast. Cut surface of the breast shows a large grey white soft fleshy tumour replacing almost whole of the breast. The tumour is somewhat delineated from the adjacent breast parenchyma (arrow) as compared to irregular margin of IDC.
Figure 25.11 
 Medullary carcinoma breast. Microscopy shows two characteristic features—large tumour cells forming syncytial arrangement and stroma infiltrated richly with lymphocytes.
Medullary carcinoma breast. Microscopy shows two characteristic features—large tumour cells forming syncytial arrangement and stroma infiltrated richly with lymphocytes.
764
|
Figure 25.12 |
Colloid (mucinous) carcinoma breast. The tumour cells |
|
|
are seen as clusters floating in pools of abundant mucin. |
||
SECTION |
3. SECRETORY (JUVENILE) CARCINOMA. This pattern |
||
extracellular PAS-positive clear spaces due to secretory |
|||
|
is found more frequently in children and has a better |
||
|
prognosis. The tumour is generally circumscribed which |
||
|
on histologic examination shows abundant intraand |
||
III |
activity of tumour cells. |
||
|
|
||
|
4. INFLAMMATORY CARCINOMA. Inflammatory |
||
Systemic |
carcinoma of the breast is a clinical entity and does not |
||
constitute a histological type. The term has been used for |
|||
|
|||
|
breast cancers in which there is redness, oedema, tenderness |
||
|
and rapid enlargement. Inflammatory carcinoma is |
||
Pathology |
associated with extensive invasion of dermal lymphatics and |
||
has a dismal prognosis. |
|||
|
|||
5. METAPLASTIC CARCINOMA. Rarely, invasive ductal carcinomas may have various types of metaplastic alterations such as squamous metaplasia, cartilagenous and osseous metaplasia, or their combinations. Development of squamous cell carcinoma of the breast parenchyma is exceedingly rare and must be separated from lesions of epidermis or nipple region.
C. PAGET’S DISEASE OF THE NIPPLE
Paget’s disease of the nipple is an eczematoid lesion of the nipple, often associated with an invasive or non-invasive ductal carcinoma of the underlying breast. The nipple bears a crusted, scaly and eczematoid lesion with a palpable subareolar mass in about half the cases. Most of the patients with palpable mass are found to have infiltrating duct carcinoma, while those with no palpable breast lump are usually subsequently found to have intraductal carcinoma. Prognosis of patients with ductal carcinoma having Paget’s disease is less favourable than of those who have ductal carcinoma without Paget’s disease.
The pathogenesis of Paget’s disease of the breast is explained by the following 2 hypotheses:
1.The tumour cells from the underlying ductal carcinoma have migrated up into the lactiferous ducts and invaded the epidermis producing skin lesions.
2.An alternate theory, though less reliable than the former, is that Paget’s disease represents a form of carcinoma in situ of the epidermis itself.
MORPHOLOGIC FEATURES. Grossly, the skin of the nipple and areola is crusted, fissured and ulcerated with oozing of serosanguineous fluid from the erosions
(Fig. 25.13, A).
Histologically, the skin lesion is characterised by the presence of Paget’s cells singly or in small clusters in the epidermis (Fig. 25.13, B). These cells are larger than the
Figure 25.13 
 Paget’s diseases of the breast. A, The region of nipple and areola is crusted and ulcerated. B, There are clefts in the epidermal layers containing large tumour cells (arrow).
Paget’s diseases of the breast. A, The region of nipple and areola is crusted and ulcerated. B, There are clefts in the epidermal layers containing large tumour cells (arrow).
epidermal cells, spherical, having hyperchromatic nuclei with cytoplasmic halo that stains positively with mucicarmine. In these respects, Paget’s cells are adenocarcinoma-type cells. In addition, the underlying breast contains invasive or non-invasive duct carcinoma which shows no obvious direct invasion of the skin of nipple.
GRADING, STAGING AND PROGNOSIS
Histologic grading and clinical staging of breast cancer determines the management and clinical course in these patients.
A. HISTOLOGIC GRADING. The breast cancers are subdivided into various histologic grades depending upon the following parameters:
1. Histologic type of tumour. Based on classification described in Table 25.1, various microscopic types of breast cancer can be subdivided into 3 histologic grades:
i)Non-metastasising—Intraductal and lobular carcinoma in situ.
ii)Less commonly metastasising—Medullary, colloid, papillary, tubular, adenoid cystic (invasive cribriform), and secretory (juvenile) carcinomas.
iii)Commonly metastasising—Infiltrating duct, invasive lobular, and inflammatory carcinomas.
2.Microscopic grade. Widely used system for microscopic grading of breast carcinoma is that of Nottingham modification of the Bloom-Richardson system. It is based on
3features:
i)Tubule formation
ii)Nuclear pleomorphism
iii)Mitotic count.
3.Tumour size. There is generally an inverse relationship between diameter of primary breast cancer at the time of mastectomy and long-term survival.
4.Axillary lymph node metastasis. Survival rate is based on the number and level of lymph nodes involved by metastasis. More the number of regional lymph nodes involved, worse is the survival rate. Involvement of the lymph nodes from proximal to distal axilla (i.e. level I— superficial axilla, to level III—deep axilla) is directly correlated with the survival rate. In this regards, identification and dissection of sentinel lymph node followed by its histopathologic examination has attained immense prognostic value (Sentinel lymph node is the first node in the vicinity to receive drainage from primary cancer i.e. it stands ‘sentinel’ over the tumour).
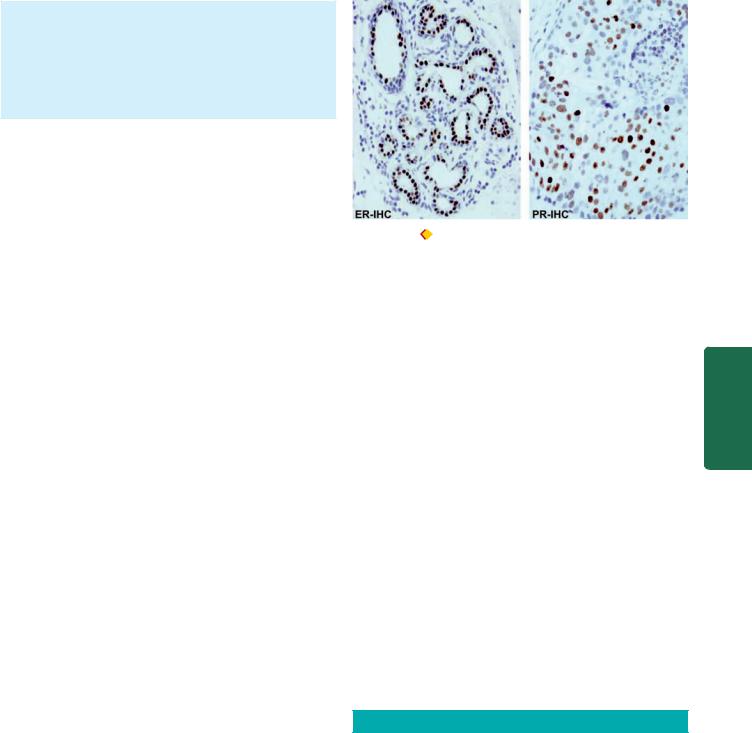
5.Oestrogen and progesterone receptors (ER/PR).
Oestrogen is known to promote the breast cancer. Presence or absence of hormone receptors on the tumour cells can help in predicting the response of breast cancer to endocrine therapy (Fig. 25.14). Accordingly, patients with high levels of ER and PR on breast tumour cells have a slightly better prognosis. A recurrent tumour that is receptor-positive is more likely to respond to anti-oestrogen therapy than one that is receptor-negative.
765
Figure 25.14 |
Oestrogen and progesterone hormonal receptors (ER |
|
|
and PR) in breast cancer. The tumour cells show nuclear positivity with |
|
||
ER and PR antibody immunostains. |
|
||
6. HER2/neu overexpression. HER2/neu (also called erbB2), |
|
||
a member of the family of epidermal growth factors, is a |
|
||
transmembrane protein having tyrosine kinase activity. It |
|
||
can be detected by immunohistochemistry or by fluorescence |
CHAPTER |
||
chemotherapy. |
|
||
in situ hybridisation (FISH) and is considered as a good |
|
||
predictive marker. An individual having overexpression of |
|
||
HER2/neu by tumour cells is likely to respond higher dose of |
|
||
herceptin therapy but is not related to other forms of |
|
||
7. DNA content. Tumour cell subpopulations with |
25 |
||
aneuploid DNA content as evaluated by mitotic markers (e.g. |
|||
|
|||
Ki-67) or by flow cytometry have a worse prognosis than |
The |
||
purely diploid tumours. |
|||
|
|||
B. CLINICAL STAGING. The American Joint Committee |
Breast |
||
(AJC) on cancer staging has modified the TNM (primary Tumour, Nodal, and distant Metastasis) staging proposed by UICC (Union International for Control of Cancer) and is shown in Table 25.2.
Spread of breast cancer to axillary lymph nodes occurs early. Later, however, distant spread by lymphatic route to internal mammary lymphatics, mediastinal lymph nodes, supraclavicular lymph nodes, pleural lymph nodes and pleural lymphatics may occur. Common sites for haematogenous metastatic spread from breast cancer are the
TABLE 25.2: AJC Clinical Staging of Breast Cancer.
Stage TIS: |
In situ carcinoma (in situ lobular, intraductal, Paget’s |
|
disease of the nipple without palpable lump) |
Stage I: |
Tumour 2 cm or less in diameter |
|
No nodal spread |
Stage II: |
Tumour > 2 cm in diameter |
|
Regional lymph nodes involved |
Stage III A: |
Tumour > 5 cm in diameter |
|
Regional lymph nodes involved on same side |
Stage III B: |
Tumour > 5 cm in diameter |
|
Supraclavicular and infraclavicular lymph nodes involved |
Stage IV: |
Tumour of any size |
|
With or without regional spread but with distant metastasis |
|
|

766
Pathology Systemic III SECTION
TABLE 25.3: Summary of Prognostic Markers and Predictive Factors for Invasive Breast Cancer.
Factor |
Favourable Prognosis |
Poor Prognosis |
|
|
|
I.ROUTINE HISTOPATHOLOGY CRITERIA:
i) |
Histologic type |
Medullary ca., tubular ca., mucinous |
Inflammatory ca. |
|
|
(colloid) ca.; lobular ca. of low grade |
|
ii) |
Tumour size (two dimensions) |
Nodal metastasis 10-20% in 1 cm |
Size larger than 1 cm |
|
|
size tumour; 10 years survival 90% |
|
|
|
in node negative |
|
iii)Histologic (Nottingham) grading
(Score range of 3-9) based on degree of tubule formation-1-3 score, regularity of nuclei-1-3 score, and mitoses-1-3 score
iv)Axillary nodal status
v)Lymphatic and/ or vascular invasion (both extratumoural)
vi)Others:
a)Tumour circumscription
b)Inflammatory reaction
c)Stromal elastosis
d)Intraductal component
e)Skin involvement
II. HORMONE RECEPTOR STATUS:
Oestrogen-progesterone receptors (ER-PR)
HER-2/neu (C-erb B-2)
III.BIOLOGICAL INDICATORS:
i)Mitotic index (by Ki67, MIB-1)
ii)DNA ploidy analysis (aneuploidy, diploidy)
Low grade (grade I) tumour = score 3-5,
moderate grade (grade II) tumour = score 6-7
Node negative: recurrence rate after 10 years 10-30%;
Number of nodes: less than 4; sentinel node negative
Negative for both: good
Good
May have some role
Absence good
Presence good
Absence good
ER-PR positive cases respond better to adjuvant therapy Under expression
Low mitotic count
Not related
High grade (grade III) tumour = score 8-9
Node positive: recurrence rate after 10 years 70%;
number of nodes: more than 4; sentinel node positive
Positive for one or both: poor
Poor
Controversial
Presence poor
Absence poor
Presence poor
ER-PR negative cases respond poorly to adjuvant therapy Over expression (predictive of response to herceptin)
High mitotic count
Not related
iii) Angiogenesis (VEGF, CD31, CD34, |
Angiogenic activity low |
High angiogenic activity |
microvessel density counts) |
|
|
iv)Oncogene disregulation
a) BRCA1, BRCA2 |
BRCA negative |
BRCA positive |
b) p53 |
p53 positive respond better to |
p53 negative respond poorly to |
|
chemotherapy and radiotherapy |
chemotherapy and radiotherapy |
c) BCL2 |
BCL2 positive good |
BCL2 negative poor |
d) Cathepsin D |
Absence indicates good prognosis |
Presence renders poor prognosis |
|
|
|
lungs, liver, bones, adrenals, brain and ovaries. Breast is one of the most suspected source of inapparent primary carcinoma in women presenting with metastatic carcinoma.
C. PROGNOSTIC FACTORS IN BREAST CANCER.
Based on current knowledge gained by breast cancer screening programmes in the West employing mammography and stereotactic biopsy, various breast cancer risk factors and prognostic factors have been described. These prognostic factors are divided into following 3 groups:
1. Potentially pre-malignant lesions. These conditions are as under:
i)Atypical ductal hyperplasia is associated with 4-5 times increased risk than women of the same age. Such lesions are commonest in the age group of 45-55 years.
ii)Clinging carcinoma is a related lesion in the duct but different from carcinoma in situ and has lower risk of progression to invasive cancer than in situ carcinoma.
iii) Fibroadenoma is a long-term risk factor (after over 20 years) for invasive breast cancer, the risk being about twice compared to controls.
2. Breast carcinoma in situ. Following factors act as determinants:
i)Ductal carcinoma in situ (comedo and non-comedo subtypes) is diagnosed on the basis of three histologic features—nuclear grade, nuclear morphology and necrosis, while lobular neoplasia includes full spectrum of changes of lobular carcinoma in situ and atypical lobular hyperplasia. Ductal carcinoma in situ is more important and demands most attention. Comedo type of in situ carcinoma has higher recurrence rate.
ii)Breast conservative therapy is used more frequently nowadays in carcinoma in situ which requires consideration of three factors for management: margins, extent of disease, and biological markers. The biological markers such as p53 and

BCL-2 have low positivity in high-grade in situ ductal carcinoma and likelihood of recurrences after conservative surgery.
3. Invasive breast cancer. Prognostic and predictive factors for invasive breast cancer have been extensively studied by univariate analysis (examining single factor separately) as well as by multivariate analysis (comparing the value of various factors included in a study). These can be broadly divided into 3 groups:
1. routine histopathology criteria; |
767 |
2.hormone receptor status; and
3.biological indicators.
A summary combining all these factors is given in
Table 25.3.
Overall, taking the most important parameter of nodepositive or node-negative breast cancer, the prognosis varies— localised form of breast cancer without axillary lymph node involvement has a survival rate of 84% while survival rate falls to 56% with nodal metastases.
Breast The 25 CHAPTER
