
- •1 Introduction and outline
- •2 Review of literature
- •2.1 Structure of dextran
- •2.2 Microbial loading in sugar factories
- •2.3 The common methods of dextran fractions determination
- •2.4 Dextran content during the process of sugar production
- •2.5 Dextrans associated with processing problems
- •2.6 Crystallization process
- •2.6.1 Growth rate of sucrose crystals
- •2.6.2 Crystallization kinetics
- •2.6.3 Parameters influencing crystallization kinetics
- •2.6.4 Crystal morphology
- •2.7 The Economic gain
- •3 Material and methods
- •3.1 Material
- •3.2 Analytical methods
- •3.2.1 Determination of dextran
- •3.2.1.1 Robert method
- •3.2.1.2 Haze method
- •3.2.2 Microbiological experiments
- •3.2.2.1 Isolation
- •3.2.2.2 Identification
- •3.2.2.2.1 Gas and acid formation
- •3.2.2.2.2 Catalase test
- •3.2.2.2.3 Gram characteristics (KOH-Test)
- •3.2.2.2.4 Identification by API 50 CHL test
- •3.2.2.2.5 L/D-Lactic acid test
- •3.2.3 Crystallization experiments
- •3.2.3.1 Measurement of growth rate of sucrose crystals
- •3.2.3.1.1 Required amount of dextran and seed
- •3.2.3.1.2 Calculation of the growth rate of sucrose crystals:
- •3.2.3.2 Dynamic viscosity
- •3.2.3.3 Crystal morphology and surface topography
- •3.2.3.4 Image analysis
- •3.2.4 Statistical analysis
- •4 Results and discussion
- •4.1 Sensitivity and accuracy of different methods for the determination of dextrans of varying molecular mass
- •4.1.1 Robert’s Copper method sensitivity
- •4.1.2 Haze method sensitivity
- •4.2 Microbial sources of dextran an identification of relevant microorganisms in sugar factories
- •4.3 Levels of dextran contents in different sugar beet factories
- •4.4 Quality of factory final products and their relationship to the levels of dextran during different industrial periods
- •4.5 Influence of dextran concentrations and molecular fractions on the rate of sucrose crystallization in pure sucrose solutions
- •4.5.1 Influence of different temperatures on growth rate of sucrose crystals in the presence of dextran
- •4.6 Elucidation of crystallization kinetics in presence of dextran molecules
- •4.7 Influence of dextran molecule fractions on sucrose solution viscosity
- •4.8 Influence of dextran on the morphology and surface topography of sucrose crystals in presence of dextran
- •4.8.1 Crystal morphology
- •4.8.2 Surface topography
- •4.9 Technical and technological consequences and future perspectives
- •5 Summary
- •6 References
- •7 Appendix
- •8 C. V. and List of Publications
Results and discussion |
86 |
4.9Technical and technological consequences and future perspectives
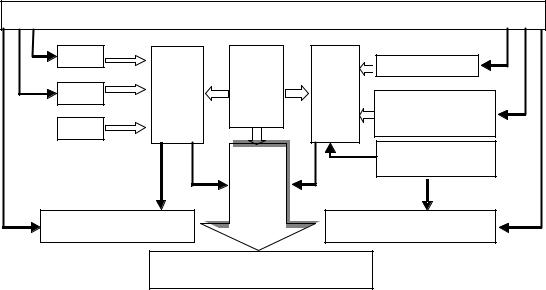
The crystallization process depends on many factors, e.g. temperature, supersaturation, solution viscosity, quantity and nature of the impurities. Dextran has a pronounced effect on the important parameters influencing the crystallization rate of sucrose solutions. Moreover, according to the achieved results it can be stated that the presence of dextran molecules in sugar syrup has a strong effect on the production and the quality of produced sugar in sugar factories. Figure 61 summarizes the most important parameters influencing the crystallization in sucrose solutions. The solution purity (q), the non-sugar content (NS), the viscosity (η) and the rate of diffusion (kD) were affected directly by the presence of dextran. The above described results confirmed that the crystallisation rate at a constant temperature is decreasing with higher dextran concentrations. At technologically relevant supersaturations and temperatures, the decrease of crystallization rate, depending on molecular weight of added dextran fractions, amounts up to 50 %.
Dextran molecules
q |
Super-Saturation |
Sucrose -Solubility |
Temperature |
Rate of diffusion |
η |
|
|||||
NS |
Relative velocity between |
||||
|
|||||
DS |
crystal and mother syrup |
||||
|
|||||
|
|
||||
|
|
|
Growth rate of sucrose crystals |
|
Stirring |
Nucleation rate |
|
|
|
Shape of crystals |
Exhaustion of mother liquor
Figure 61: Influence of dextran on many crystallization parameters during crystallization process.
Where: q = purity, NS = non-sugar, DS = dry substance and η = viscosity
Time reduction and low energy consumption in the crystallization process are of particular interest in sugar factories. Figure 62 shows time periods for the reduction of dry mass content by one percent for different dextran concentrations at 60°C and 70°C, respectively. At 60°C the crystallization periods were increased by 20 % and

Results and discussion |
87 |
68 % after the addition of dextran T2000 at concentrations of 1500 and 5000 mg/kg DS, respectively. On the other hand, at 70°C the increase of crystallization time was 10 % and 21 %. Furthermore, an increase of crystallization temperature from 60°C to 70°C reduces the crystallization time by 8 %, 16 % and 33 % regarding admixtures of dextran of 0 ppm, 1500 ppm or 5000 ppm, respectively. Moreover, the influence of dextran increases strongly with both, the increase of molecular weight and the decrease of crystallization temperature. Therefore, it is obvious, that a decrease in crystallization temperature, e.g. from 70°C to 65°C, which is desirable regarding energy aspects, is not applicable in occurrence of dextran. Technological measures to manipulate only the high molecular fractions of dextran are not known so far.
|
2.5 |
|
|
68% |
|
|
|
|
|
|
2 |
20% |
|
|
(hours) |
|
21% |
||
|
|
|
||
1.5 |
|
10% |
|
|
|
|
|
||
time |
|
|
|
|
Crystallization |
1 |
|
|
|
0.5 |
|
|
|
|
|
|
|
|
|
|
0 |
|
|
|
|
0 mg/kg DS |
1500 mg/kg DS |
5000 mg/kg DS |
|
|
|
Dextran content (mg/kg DS) |
|
|
|
|
60°C |
70°C |
|
Figure 62: The relationship between dextran additions and crystallization time during the crystallization process
In order to reach similar crystallization rates at the occurrence of dextran higher supersaturations can be applied. However, the increase of supersaturation above a certain level may cause spontaneous crystallization and thus the formation of fine crystals. In practice, sugar factories have to work in the so called metastable zone from 1 to 1.2 supersaturation (Brennan et al., 1976; van der Poel et al., 1998). Thus, narrow boundaries are set considering an increase of supersaturation.
Results and discussion |
88 |
Basing on the abovementioned results future research topics are suggested on the following topics:
¾Study on the effect of other organic non-sugar substances (e.g. other macromolecular polysaccharide fractions or proteins) on sugar process productions and sugar quality. Rogé et al. (2007) reported that organic macromolecules act as carrier for non-sugar substances causing problems during crystallization and causing turbidity in the final sugar. This effect was also proved for dextran in this work. However, no comprehensive work is known regarding the composition of organic macromolecules occurring during sugar production.
¾In chapter 4.3 and 4.8 the relation between dextran concentration in syrup and dextran concentration in sugar was illustrated. It was mentioned that this relation is dependent on the crystallization and centrifugation regime. In order to allow a precise process control to minimize the negative impact of dextran a systematic investigation about the influence of relevant process parameters (e.g. number of crystallization stages, amount of water for affination, type of sugar production factory) on the transfer of dextran into the sugar has to be realized. A qualitative and quantitative evaluation by determination of dextran levels in the after product is suggested. This could be a valuable tool to supplement available model based process control tools in the sugar industry (Fiedler, et. al. 2007).
¾Based on the described effects of dextran on the crystal surface (s. chapter 4.8.2), future studies will investigate the relationship between dextran levels and especially the drying process in order to characterize parameters influencing the formation of micro-crystals and the incorporation of microparticles (colorants and turbidity) on the crystal surface.
¾Improvement of crystallization process at low temperatures (comp. chapter 4.6) by decreasing surface tension and viscosity using chemical agents during the crystallization process.
¾Usage of enzyme (dextranase) during the extraction process of sugar beet juices to improve the filtration process (Clarke,1997). The latter leads to an increase of the soluble dextran amount (low molecular mass) in juice. This means an increase of dextran levels in syrup and, hence, an increase of low molecular weight dextran. The low molecular weight fraction of dextran is
Results and discussion |
89 |
known to affect the crystallization process and the sugar loss to molasses (needle crystals), which was proved in the present study (see chapter 4.8.1). Furthermore, the enzyme treatment leads to increase of glucose, which causes an increase of color formation during the evaporation process (Maillard reaction) (Kroh, et. al. 2007). In addition, it causes a reduction of crystallization rate. The future work will focus on dextran molecular weight fractions and sugars deriving from enzyme treatment and on the effect of these products on the sugar production process.
