
- •Table of Contents
- •Preface
- •Contributors
- •1. INTRODUCTION
- •2. HIERARCHIES OF AB INITIO THEORY
- •2.3. Computational Cost
- •3.2. The CCSD(T) Model
- •4.1. Electronic and Nuclear Contributions
- •4.2. Dependence on the AO Basis Set
- •5.2. Extrapolations from Principal Expansions
- •6. CALIBRATION OF THE EXTRAPOLATION TECHNIQUE
- •6.2. Total Electronic Energy
- •6.3. Core Contributions to AEs
- •7. MOLECULAR VIBRATIONAL CORRECTIONS
- •8. RELATIVISTIC CONTRIBUTIONS
- •9. CALCULATION OF ATOMIZATION ENERGIES
- •10. CONCLUSIONS AND PERSPECTIVES
- •2. STEPS IN THE W1 AND W2 THEORIES, AND THEIR JUSTIFICATION
- •2.1. Reference Geometry
- •2.2. The SCF Component of TAE
- •2.3. The CCSD Valence Correlation Component of TAE
- •2.4. Connected Triple Excitations: the (T) Valence Correlation Component of TAE
- •2.6. Scalar Relativistic Correction
- •3. PERFORMANCE OF W1 AND W2 THEORIES
- •3.2. Electron Affinities (the G2/97 Set)
- •3.4. Heats of Formation (the G2/97 Set)
- •3.5. Proton Affinities
- •4. VARIANTS AND SIMPLIFICATIONS
- •4.2. W1h and W2h Theories
- •4.5. W1c Theory
- •4.6. Detecting Problems
- •5. EXAMPLE APPLICATIONS
- •5.1. Heats of Vaporization of Boron and Silicon
- •5.2. Validating DFT Methods for Transition States: the Walden Inversion
- •5.3. Benzene as a ”Stress Test” of the Method
- •6. CONCLUSIONS AND PROSPECTS
- •1. INTRODUCTION
- •2. THE G3/99 TEST SET
- •4. G3S THEORY
- •5. G3X THEORY
- •6. DENSITY FUNCTIONAL THEORY
- •7. CONCLUDING REMARKS
- •1. INTRODUCTION
- •2. PAIR NATURAL ORBITAL EXTRAPOLATIONS
- •3. CURRENT CBS MODELS
- •4. TRANSITION STATES
- •5. EXPLICIT FUNCTIONS OF THE INTERELECTRON DISTANCE
- •7. NEW DEVELOPMENTS
- •7.1. The SCF Limit
- •7.2. The CBS Limit for the MP2 Correlation Energy
- •7.4. Total Energies
- •8. ENZYME KINETICS AND MECHANISM
- •9. SUMMARY
- •1. INTRODUCTION
- •2. ELECTRON PROPAGATOR CONCEPTS
- •3. AN ECONOMICAL APPROXIMATION: P3
- •4. OTHER DIAGONAL APPROXIMATIONS
- •5. NONDIAGONAL APPROXIMATIONS
- •7. P3 TEST RESULTS
- •7.1. Atomic Ionization Energies
- •7.2. Molecular Species
- •8. CONCLUSIONS AND PROSPECTUS
- •1. INTRODUCTION
- •2. THEORETICAL PROCEDURES
- •3. GEOMETRIES
- •4. HEATS OF FORMATION
- •5. BOND DISSOCIATION ENERGIES
- •6. RADICAL STABILIZATION ENERGIES
- •7. REACTION BARRIERS
- •8. REACTION ENTHALPIES
- •9. CONCLUDING REMARKS
- •1. INTRODUCTION
- •2. HOMOLEPTIC CARBONYL COMPLEXES
- •4. IRON CARBONYL COMPLEXES
- •5. GROUP-10 CARBONYL COMPLEXES
- •7. NOBLE GAS COMPLEXES
- •8. TRANSITION METAL CARBENE AND CARBYNE COMPLEXES
- •12. TRANSITION METAL METHYL AND PHENYL COMPOUNDS
- •13. TRANSITION METAL NITRIDO AND PHOSPHIDO COMPLEXES
- •15. MAIN GROUP COMPLEXES OF BeO
- •16. CONCLUSION
- •1. INTRODUCTION
- •2. THEORETICAL BACKGROUND
- •3. SPECIFIC CONVENTIONS
- •4. STATISTICAL EVALUATIONS
- •5. DISCUSSION
- •Index
118 |
Chapter 4 |
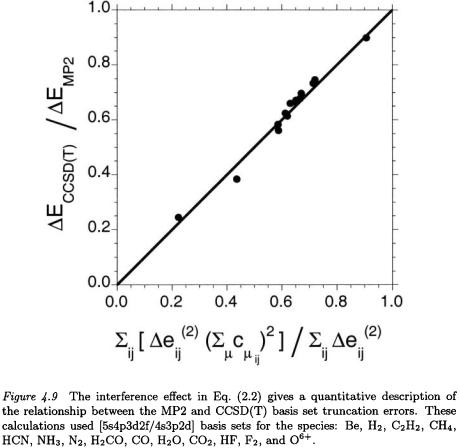
(2.1). This CBS extrapolation reduces the errors in the cc-pVQZ and ccpV5Z higher-order correlation energy by an order of magnitude (Table 4.7), but seriously over-corrects the cc-pVDZ and cc-pVTZ higher-order energies [50]. A simple scaling to reduce the CBS correction to the ccpVDZ and cc-pVTZ energies reduces the RMS errors below 1 kcal/mol for both (Table 4.7). This single adjustable CBS higher-order parameter might be compared to the use of a single adjustable parameter in the W1 theory [55].
7.4.Total Energies
Having established that size-consistent extrapolations of energies obtained with the cc-pVDZ and cc-pVTZ basis sets are capable of producing sub-kcal/mol absolute accuracy for SCF energies (Table 4.5),
Complete Basis Set Models |
119 |
MP2 correlation energies (Table 4.6), and the higher-order contributions to the correlation energy (Table 4.7), we can now combine these components to obtain total electronic energies. There are many plausible combinations of basis sets and extrapolation procedures that must ultimately be explored. Efficient methods should use smaller basis sets for the CCSD(T) component than for the SCF and MP2 ones. The use of intermediate basis sets for the MP4(SDQ) component should also be explored, since we found this effective for the CBS-QB3 model (Table 4.2).
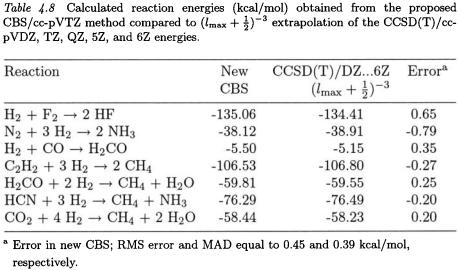
As a first try, we have elected to follow our treatment of the SCF and second-order correlation energies described above, and employ Eq. (6.2) to provide a linear extrapolation of the cc-pVDZ and cc-pVTZ total CBS-CCSD(T) energies obtained with Eq. (2.2), including the interference correction. These total energies reproduce the CCSD(T) limits estimated by Martin [55] via an  extrapolation of the CCSD(T)/cc-pVDZ, TZ, QZ, 5Z, and 6Z basis sets to within 0.96 kcal/mol RMS error. The agreement with Martin’s energies for a small set of chemical reactions is even better (Table 4.8). The use of the ccpVnZ basis sets for
extrapolation of the CCSD(T)/cc-pVDZ, TZ, QZ, 5Z, and 6Z basis sets to within 0.96 kcal/mol RMS error. The agreement with Martin’s energies for a small set of chemical reactions is even better (Table 4.8). The use of the ccpVnZ basis sets for  double extrapolations is indeed promising.
double extrapolations is indeed promising.
