
6. Mass spectrometry of nitro and nitroso compounds |
289 |
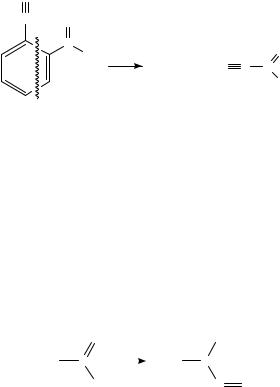
ionized benzonitrile108. Thus, a mechanism involving the elimination of nitroacetylene was proposed108; see Scheme 39.
N
CO
N +
OH |
+ |
O |
|
+ HC C N |
|||
|
C4 H4 N |
||
|
|
O |
SCHEME 39
Direct analysis of complex samples have revealed neutral losses as most useful5. Thus, nitroarenes have been identified based on the losses of OHž from the [M C H]C ions109. However, the fact that some isomers will be detected with a very low sensitivity, if at all, puts a strong limitation to this strategy.
Protonated aliphatic/alicyclic nitro compounds have not been studied to the same extent as the nitroarenes. However, the isomeric [C2H6NO2]C ions and their rearrangement in the gas phase have been subject to studies110. The protonated ethyl nitrite and nitroethane were generated by proton transfer from different Brønsted acids. In addition, protonated ethyl nitrite was obtained as a result of addition of NOC to ethanol110. The existence of two different [C2H6NO2]C structures, i.e. protonated ethyl nitrite and protonated nitroethane, was demonstrated. Slow isomerization of protonated nitroethane to protonated ethyl nitrite has been elucidated110; see Scheme 40.
O |
|
H |
|
+ |
|
C2 H5 N |
|
C2 H5 O |
|
||
+ |
|
N O |
OH |
|
|
SCHEME 40
The chemical ionization of nitro compounds has been reported to cause chemical transformation of the analyte. Thus, reduction of the nitro group to the corresponding amine appears as a common process. The extent of reduction depends on the ion source temperature and the possible presence of water in the system. It should be noted that the protonated amine and the [MH NO]C ion are isobaric and the possible differentation can be achieved applying either high resolution or isotopical shift techniques. With the use of H2O, respectively D2O, as reagent gases, it was demonstrated that [MH 30]C ions mainly were due to the reduction of the compounds to the corresponding amines111.
The use of ammonia for the protonation of nitroarenes leads frequently to formation of aduct ions, e.g. [M C NH4]C , but not to the protonated species MHC 112,113. The ammonia chemical ionization spectrum of nitrobenzene shows, in addition to a series of adduct ions, a dominant signal corresponding to the anilinium ion m/z 94 112,114,115. Evidence for the isomerization of the [M C NH4]C adduct followed by successive loss of NO and OHž or NH3 to give ions corresponding to the substitution products, e.g. the anilinium ion, has been given115; see Scheme 41.
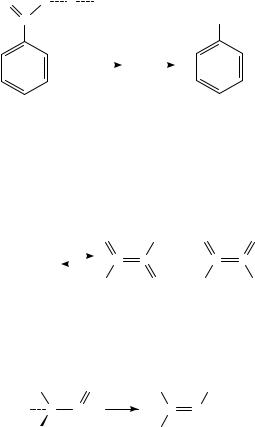
290 |
Helge Egsgaard and Lars Carlsen |
|
||||
|
+ |
|
|
|
|
|
O |
O H |
NH3 |
+ |
|||
N |
|
|
|
|
|
NH3 |
|
|
|
|
|
|
|
SCHEME 41
V. NITROSO COMPOUNDS
Mass spectrometric investigations of C-nitroso compounds are rarely encountered in the literature2. C-nitroso compounds are difficult to handle due to dimerization and isomerization. Dimerization is a common feature leading to a dimer exhibiting cis/trans isomeric forms; see Scheme 42.
|
|
|
|
|
|
|
O |
R |
O |
O |
2 R |
|
N |
|
O |
|
|
|
N N |
and/or N |
N |
|
|
|
||||||||
|
|
|||||||||
|
||||||||||
|
|
|||||||||
|
|
|
|
|
|
|
R |
O |
R |
R |
SCHEME 42
C-nitroso compounds exhibiting an ˛-H, such as nitrosomethane and nitrosoethane, may isomerize to the corresponding oxime, as evidenced by mass spectrometry116,117. Unsaturated C-nitroso compounds may undergo electrocyclic ring closure as discussed below.
H |
O |
R |
OH |
R C |
N |
C |
N |
R |
|
R |
|
Consequently, the MS investigations of C-nitroso compounds are frequently hampered by superimposed spectra due to dimerization/rearrangement of the initial compound. Further, electron impact spectra of larger non-aromatic C-nitroso compounds in general carry little information due to extensive fragmentation118.
A study of a series of C-nitroso compounds, including monomers as well as dimers, by field desorption has demonstrated the superiority of this technique to this class of compounds118. All the compounds display intense molecular ions118. The method has a significant potential for studies of the equilibrium between mixed and pure C-nitroso compounds, since the amount of pure and mixed dimers present in a solution apparently can be visualized by the relative abundances of the respective molecular ions; see Scheme 43. Determination of the concentrations versus time may resolve the kinetics of the dimer formation118.
The parent nitrosoethene has been characterized by MS strategies45. The compound was generated by low-pressure pyrolysis using surface-promoted reactions of nitroethene45. The MS reveal that nitrosoethene undergoes a simple cleavage. The charge predominantly remaining on the C2H3 fragment was unambiguously demonstrated by application of deuterium labelling45. Thus, the isotope shifts observed in the collision-induced MS of
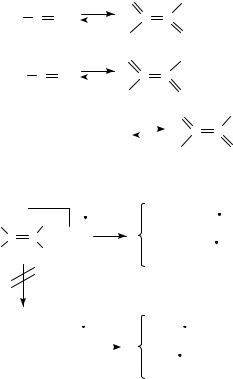
6. Mass spectrometry of nitro and nitroso compounds |
291 |
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
R1 |
|
|||
2 |
|
R1 |
N |
O |
|
|
|
|
|
R1 |
N |
N |
|
cis/trans |
|
||||
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
||||
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
R2 |
|
|||
2 |
|
R2 |
N |
O |
|
|
|
|
|
R2 |
N |
N |
O |
cis/trans |
|
||||
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
R2 |
|
R1 |
|
N |
|
O + R2 |
|
|
N |
|
O |
|
|
|
|
R1 |
N N |
|
|||
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
||
|
|
|
|
|
|
|
SCHEME 43 |
|
|
|
|
|
|
||||||
H |
|
|
NO |
+ |
|
|
|
|
|
|
|
|
C2 H3 |
+ |
+ NO |
|
|||
|
|
C |
C |
|
|
|
|
|
|
|
|
|
|
NO+ |
+ |
|
|
||
H |
|
|
H |
|
|
|
|
|
|
|
|
|
|
C2 H3 |
|
||||
O |
|
|
|
N |
+ |
|
|
CH2 O+ + HCN |
|||
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
HCN+ + CH2 O |
|
|
|
|
|
|
|
|
|
|
|
|
H2 C |
|
|
|
|
CH |
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
SCHEME 44 |
||
the pyrolytically generated compound apparently exclude the operation of [2 C 2 ] intramolecular cycloaddition, since it may be expected that the ionized isomeric 4-H-1,2- oxazete fragments to HCNCž and CH2OCž119 ; see Scheme 44.
VI. REFERENCES
1.C. J. Porter, J. H. Beynon and T. Ast, Org. Mass Spectrom., 16, 101 (1981).
2.H. Schwarz and K. Levsen, in Chemistry of the Functional Groups, Suppl. F: The Chemistry of Amino, Nitroso and Nitro Compounds and Their Derivatives, Part 1, (Ed. S. Patai), Chap. 3, Wiley, Chichister 1982, p. 85.
3.K. Levsen, Fundamental Aspects of Organic Mass Spectrometry, Verlag Chemie, Weinheim, 1978.
4.R. G. Cooks, J. H. Beynon, R. M. Caprioli and G. R. Lester, Metastable Ions, Elsevier, Amsterdam, 1973.
5.K. L. Busch, G. L. Glish and S. McLuckey, Mass Spectrometry/Mass Spectrometry: Techniques and Applications of Tandem Mass Spectrometry, VCH Verlagsgesellschaft, Weinheim, 1988.
6.H. Egsgaard, L. Carlsen, H. Florencio,ˆ T. Drewello and H. Schwarz, unpublished.
7.C. Lifshitz, M. Rejwan, I. Levin and T. Peres, Int. J. Mass Spectrom. Ion Processes, 84, 271 (1988).
8.J. L. Holmes and J. K. Terlouw, Org. Mass Spectrom., 15, 383 (1980).
9.A. Fraefel and L. Seibl, Mass Spectrom Rev., 4, 151 (1985).
10.R. K. Boyd and J. H. Beynon, Int. J. Mass Spectrom. Ion Phys., 23, 163 (1977).
11.J. H. Beynon, M. Bertrand and R. G. Cooks, J. Am. Chem. Soc., 95, 1739 (1973).
292 |
Helge Egsgaard and Lars Carlsen |
12.K. Levsen and H. Schwarz, Mass Spectrom. Rev., 2, 77 (1983).
13.F. W. McLafferty (Ed.), Tandem Mass Spectrometry, Wiley-Interscience, New York, 1983.
14.F. W. McLafferty, Acc. Chem. Res., 13, 33 (1980).
15.H. Egsgaard, L. Carlsen, H. Florencio,ˆ T. Drewello and H. Schwarz, Ber. Bunsenges. Phys. Chem., 93, 76 (1989).
16.C. Wesdemiotis and F. W. McLafferty, Chem. Rev., 87, 485 (1987).
17.J. K. Terlouw and H. Schwarz, Angew. Chem., 99, 829 (1987).
18.J. K. Terlouw, Adv. Mass Spectrom., 11B, 984 (1989).
19.J. L. Holmes, Mass Spectrom. Rev., 8, 513 (1989).
20.F. W. McLafferty, Science, 247, 925 (1990).
21.F. Turecek, Org. Mass Spectrom., 27, 1087 (1992).
22.A. W. McMahon, F. Chadikun, A. G. Harrison and R. E. March, Int. J. Mass Spectrom. Ion Processes., 87, 275 (1989).
23.P. C. Burgers and J. K. Terlouw, Structures and Reactions of Gas-phase Organic Ions, Special Periodic Report, Vol. 10, Royal Society of Chemistry, 1989, p. 35.
24.S. G. Lias, J. E. Bartmess, J. F. Liebman, J. L. Holmes, R. D. Levin and W. G. Mallard, J. Phys. Chem. Ref. Data, 17, suppl 1 (1988).
25.J. L. Holmes, M. Fingas and F. P. Lossing, Can. J. Chem., 59, 80 (1981).
26.J. L. Holmes and F. P. Lossing, Can. J. Chem., 60, 2365 (1982).
27.J. L. Holmes, F. P. Lossing and P. C. Burgers, Int. J. Mass Spectrom. Ion Phys., 47, 133 (1983).
28.F. P. Lossing and J. L. Holmes, J. Am. Chem. Soc., 106, 6917 (1984).
29.H. Egsgaard and L. Carlsen, unpublished.
30.J. P. Gilman, T. Hsieh and G. G. Meisels, J. Chem. Phys., 78, 1174 (1983).
31.J. W. Rabalias, J. Chem. Phys., 57, 960 (1972).
32.M. L. McKee, J. Phys. Chem., 90, 2335 (1986).
33.M. L. McKee, J. Am. Chem. Soc., 108, 5784 (1986).
34.P. Kebarle and S. Chowdhury, Chem. Rev., 87, 513 (1987).
35.T. Heinis, S. Chowdhury and P. Kebarle, Org. Mass Spectrom., 28, 358 (1993).
36.R. J. Cellota, R. A. Bennet and J. L. Hall, J. Chem. Phys., 60, 1740 (1974).
37.G. W. Dillow and P. Kebarle, J. Am. Chem. Soc., 111, 5592 (1989).
38.S. Chowdhury, H. Kishi, G. W. Dillow and P. Kebarle, Can. J. Chem., 67, 603 (1989).
39.S. G. Lias, J. F. Liebman and R. D. Levin, J. Phys. Chem. Ref. Data, 13, 695 (1984).
40.D. P. Stevenson, Discuss. Faraday Soc., 10, 35 (1951).
41.Y. Niwa, S. Tajima and T. Tsuchiya, Int. J. Mass Spectrom. Ion Phys., 40, 287 (1981).
42.M. Panczel and T. Baer, Int. J. Mass Spectrom. Ion Processes, 58, 43 (1984).
43.T. Nishimura, P. R. Das and G. G. Meisels, J. Chem. Phys., 84, 6190 (1986).
44.H. Egsgaard and L. Carlsen, Proc. 41st ASMS Conf. Mass Spectrom. Allied Topics, San Francisco, May 1993, p. 993a.
45.H. Egsgaard and L. Carlsen, J. Chem. Res. (S), 18 (1987).
46.H. Egsgaard, L. Carlsen, T. Weiske, D. Sulzle¨ and H. Schwarz, Chem. Phys. Lett., 199, 643 (1992).
47.G. Bouchoux, Mass Spectrom. Rev., 7, 1 (1988).
48.G. Bouchoux, Mass Spectrom. Rev., 7, 203 (1988).
49.H. Schwarz, Adv. Mass Spectrom. 1985, Part A (Ed. J. F. Todd), p 13 (1986).
50.H. Egsgaard, L. Carlsen and S. Elbel, Ber. Bunsenges. Phys. Chem., 90, 369 (1986).
51.S. K. Hindawi, R. H. Fokkens, F. A. Pinkse and N. M. M. Nibbering, Org. Mass Spectrom., 21, 243 (1986).
52.M. Sirois, J. L. Holmes and C. E. C. A. Hop, Org. Mass Spectrom., 25, 167 (1990).
53.R. Arakawa, J. Bull. Chem. Soc. Jpn., 62, 2064 (1989).
54.M. P. Irion, A. Selinger, A. W. Castleman Jr., E. E. Ferguson and K. G. Weil, Chem. Phys. Lett., 147, 33 (1988).
55.H. Egsgaard and L. Carlsen, Chem. Phys. Lett., 147, 30 (1988).
56.J. P. Gilman, T. Hsieh and G. G. Meisels, J. Chem. Phys., 78, 3767 (1983).
57.D. Schroder,¨ D. Sulzle,¨ O. Dutuit, T. Baer and H. Schwarz, J. Am. Chem. Soc., 116, 6395 (1994).
58.S. Tajima, T. Azami, T. Tsuchiya and N. M. M. Nibbering, Sh. Bunseki, 31, 51 (1983).
59.N. M. M. Nibbering Th. J. de Boer and H. J. Hofman, Recl. Trav. Chim. Pays-Bas, 84, 481 (1965).
60.J. W. Verhoeven, Recl. Trav. Chim. Pays-Bas, 99, 369 (1980).
6. Mass spectrometry of nitro and nitroso compounds |
293 |
61.H. Egsgaard and L. Carlsen, Org. Mass Spectrom., 24, 1031 (1989).
62.H. Schwarz, Top. Curr. Chem., 73, 231 (1978).
63.J. H. Beynon, G. R. Lester and A. E. Williams, J. Phys. Chem., 63, 1861 (1959).
64.A. R. Butcher and C. B. Thomas, Org. Mass Spectrom., 14, 448 (1979).
65.J-D Shao and Tomas Baer, Int J. Mass Spectrom and Ion Processes., 86, 357 (1988).
66.S. A. McLuckey and G. L. Glish, Org. Mass. Spectrom., 22, 224 (1987).
67.C. G. Herbert, E. A. Larka and J. H. Beynon, Org. Mass Spectrom., 19, 306 (1984).
68.S. Meyerson, I. Puskas and E. K. Fields, J. Am. Chem. Soc., 88, 4974 (1966).
69.J. H. Beynon, R. A. Saunders, A. Topham and A. E. Williams, J. Chem. Soc., 6403 (1965).
70.M. A. Baldwin, Org. Mass Spectrom., 15, 109 (1980).
71.M. A. Baldwin, D. M. Carter and J. Gilmore, Org. Mass Spectrom., 17, 45 (1982).
72.M. A. Baldwin and H. J. Bowley, Org. Mass Spectrom., 17, 580 (1982).
73.T. G. Morgan, E. E. Kingston, F. M. Harris and J. H. Beynon, Org. Mass Spectrom., 17, 594 (1982).
74.C. G. Macdonald and M. J. Lacey, Org. Mass Spectrom., 17, 91 (1982).
75.K. B. Tomer, T. Gebreyesus and C. Djerassi, Org. Mass Spectrom., 7, 383 (1973).
76.C. J. Porter, C. J. Proctor, E. A. Larka and J. H. Beynon, Org. Mass Spectrom., 17, 331 (1982).
77.G. Depke, W. Klose and H. Schwarz, Org. Mass Spectrom., 18, 495 (1983).
78.S. Midleton, M. Butcher and R. F. Mathews, Aust. J. Chem., 27, 2583 (1974).
79.G. Depke, W. Klose, H. Schwarz, W. Blum and W. J. Richter, Org. Mass Spectrom., 18, 568 (1983).
80.R. K. M. R. Kallury and J. Hemalatha, Org. Mass Spectrom., 15, 659 (1980).
81.D. V. Ramana and N. V. S. Rama Krishna, Org. Mass Spectrom., 24, 66 (1989).
82.D. V. Ramana, K. K. Balasubramanian, M. S. Sudha and T. Balasubramanian, J. Am. Soc. Mass Spectrom., 6, 195 (1995).
83.P. S. Kulkarni, H. V. Kamath and S. N. Kulkarni, Org. Mass Spectrom., 19, 334 (1984).
84. A. Konnecke,¨ P. Lepom, R. Dorre,¨ E. Liepmann, R. Herzshuh, B. Kralj, B. Stanovnik and
M. Tisler, Org. Mass Spectrom., 15, 75 (1980).
85.A. Maquestiau, R. Flammang, M. Flammang-Barbieux, Lin-Zhi Chen and C. Wentrup, Int. J. Mass Spectrom and Ion Processes, 86, 235 (1988).
86.D. V. Ramana, N. Sundaram and M. George, Org. Mass Spectrom., 24, 63 (1989).
87.D. V. Ramana, N. Sundaram and M. George, Org. Mass Spectrom., 25, 161 (1990).
88.D. V. Ramana and N. V. S. Rama Krishna, Org. Mass Spectrom., 27, 515 (1992).
89.D. V. Ramana and N. V. S. Rama Krishna, Org. Mass Spectrom., 24, 485 (1989).
90.D. V. Ramana, N. Sundaram and M. George, Org. Mass Spectrom., 22, 140 (1987).
91.D. V. Ramana and S. K. Viswanadham, Org. Mass Spectrom., 18, 162 (1983).
92.J. Yinon and S. Bulusu, Org. Mass Spectrom., 21, 529 (1986).
93.S. Bulusu and T. Axenrod, Org. Mass Spectrom., 14, 585 (1979).
94.W. R. Carper, R. C. Dorey, K. B. Tomer and F. W. Crow, Org. Mass Spectrom., 19, 623 (1984).
95.J. Yinon, Org. Mass Spectrom., 27, 689 (1992).
96.W. C. M. M. Luijten and J. van Thuijl, Org. Mass Spectrom., 14, 577 (1979).
97.W. C. M. M. Luijten and J. van Thuijl, Org. Mass Spectrom., 16, 199 (1981).
98.W. C. M. M. Luijten and J. van Thuijl, Org. Mass Spectrom., 17, 299 (1982).
99.W. C. M. M. Luijten and J. van Thuijl, Org. Mass Spectrom., 17, 304 (1982).
100.A. Cert, P. Delgado-Cobos and M. T. Perez´-Lanzac, Org. Mass Spectrom., 21, 499 (1986).
101.D. Mitchell, R. D. Bowen, K. R. Jennings, R. S. Varma and G. W. Kalbalka, J. Chem. Soc., Perkin Trans. 2, 1495 (1987).
102.S. R. Deshpande, H. M. Mathur and G. K. Trivedi, Ind. J. Chem., 24B, 1142 (1985).
103.R. Gawinecky, D. Rasala and T. Bak, Org. Mass Spectrom., 27, 39 (1992).
104.D. V. Ramana and P. Mahalakshmi, Org. Mass Spectrom., 28, 107 (1993).
105.S. Pappalardo and M. Di Grazia, Org. Mass Spectrom., 20, 392 (1985).
106.A. G. Harrison and R. K. M. R. Kallury, Org. Mass Spectrom., 15, 284 (1980).
107.Y. H. Lau and P. Kebarle, J. Am. Chem. Soc., 98, 7452 (1976).
108.R. A. Crombie and A. G. Harrison, Org. Mass Spectrom., 23, 327 (1988).
109.D. Schuetzle, T. L. Riley, T. J. Prater, T. M. Harvey and D. F. Hunt, Anal. Chem., 54, 265 (1982).
110.G. de Petris, Org. Mass Spectrom., 25, 557 (1990).
111.J. Yinon and M. Laschever, Org. Mass Spectrom., 16, 264 (1981).
294 |
Helge Egsgaard and Lars Carlsen |
112.R. G. Gillis, Org. Mass Spectrom., 21, 415 (1986).
113.A. G. Harrison, Chemical Ionization Mass Spectrometry, 2nd ed., CRC Press, Boca Raton, 1992.
114.W. J. van der Hart, W. C. M. M. Luijten and J. van Thuijl, Org. Mass Spectrom., 15, 463 (1980).
115.K. G. Das, R. A. Swamy, J. van Thuijl, W. Onkenhout, D. Fraisse and J. C. Tabet, Org. Mass Spectrom., 18, 34 (1983).
116.D. C. Frost, W. M. Lau, C. A. McDowell and N. P. C. Westwood, J. Phys. Chem., 86, 3577 (1982).
117.G. B. M. Eastwood and G. L. Pratt, J. Chem. Soc. (A), 2337 (1970).
118.R. L. Johnson, O. W. Lever Jr. and D. A. Brent, Org. Mass Spectrom., 118, 36 (1983).
119.K. Wieser and A. Berndt, Angew. Chem., 87, 72 (1975).
