

6. Mass spectrometry of nitro and nitroso compounds |
259 |
In addition, the ion chemistry of nitro compounds following protonation as well as that of negative ions will be discussed.
B. Simple Cleavages
Nitro compounds may show two different simple cleavages with respect to the nitro functionality, i.e. cleavage of the C N and N O bond, respectively.
|
|
|
|
|
|
|
|
|
+ |
|
|
|
R+ + |
NO2 |
|||||
R |
|
|
|
NO2 |
|
|
|
|
R + +NO2 |
||||||||||
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
+ |
R |
|
NO2 |
|
|
|
|
|
|
R |
|
NO |
|
|
+ Ο |
|||||
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
predicts comparable ion intensities near the thermodynamic onset for CH3C and NO2C produced by a C N bond rupture of the nitromethane radical cation since CH3C and NO2C are close in ionization energies. However, the difference between the dissociation limit and the observed appearence energy amounts to 1.2 eV41. The energy release with photon energy of 14.73 eV was 0.37 eV and 0.24 eV for NO2C and CH3C , respectively41. Thus, it was concluded that either CH3C or NO2 was formed in an electronically excited state rather than in an electronic ground state41. This suggests that the CH3C may arise from a specific excited molecular ion41.
The cleavage of the C N bond in the nitrobenzene radical cation, on the other hand, gives rise to the C6H5C ion. This reaction has been studied by PEPICO and, based on fragmentation rates, it was concluded that the NO2 loss most likely takes place from an electronically excited state42. However, a later PEPICO study revealed that the dependence of the fragmentation rate constants on internal energy is in good agreement with the RRKM/QET calculations assuming a slightly tight transition state43. In addition, the average kinetic energy release was in good quantitative agreement with that calculated by statistical phase space theory43.
Electron impact mass spectra of nitro compounds show generally [M O]Cž ions, however, in low intensity. Several [M O]Cž ions, e.g. the [M 16]Cž of nitromethane44 and nitroethene45, has by tandem mass spectrometry unambiguously been demonstrated to be of the nitroso form. Particularly interesting is the use of the parent nitramide in NRMS study of the elusive nitrosamide46.
+ − O |
+ + e− |
|
− e− |
+ |
||
NH2 NO2 |
|
NH2 NO |
|
|
|
NH2 NO |
|
|
NH2 NO |
||||
|
|
|
|
|
||
Neutralization - Reionization
C. Rearrangements
1. Tautomerism: Nitro/aci-nitro radical cations
The fragmentation characteristics of the CH3NO2 isomers have been studied by a number of investigators and the system constitutes one of the most well-decribed within the field of mass spectrometry. Apparently intricate inter-relations between the isomers do exist.

260 |
|
Helge Egsgaard and Lars Carlsen |
|
|
|
||||
|
|
+ |
|
|
|
|
|
|
|
|
O |
O |
|
|
|
|
|
+ |
|
|
+ |
|
|
|
|||||
|
|
CH3 |
|
O |
|
NO |
|||
|
|
|
|||||||
CH2 N |
CH3 N |
|
|
|
|||||
|
|
|
|||||||
|
OH |
|
O |
|
|
|
|||
The aci-form of the nitro group is frequently claimed in pure chemistry. However, only aci-nitromethane appears to have been comprehensively studied as an isolated species15. Ionized keto enol systems are characterized by reversal of the relative stabilities of the single species compared to their neutral counterparts. Thus, the ionized enols are generally the thermodynamically more favoured tautomers by approximately 15 20 kcal mol 1, the
two tautomers being separated by a significant barrier of ca 50 kcal mol 147 49 .
The aci-nitromethane radical ion may be generated by a facile loss of ethylene from the radical cation of 1-nitropropane via a 1,5-hydrogen shift15,50,51.
H |
H |
|
|
O |
+ |
|
|
O |
|
|
|
||
|
|
|
|
|
||
H |
|
−C2 |
H4 |
CH2 |
N |
|
+N |
|
|
||||
|
|
|
|
|
||
|
O |
|
|
OH |
|
|
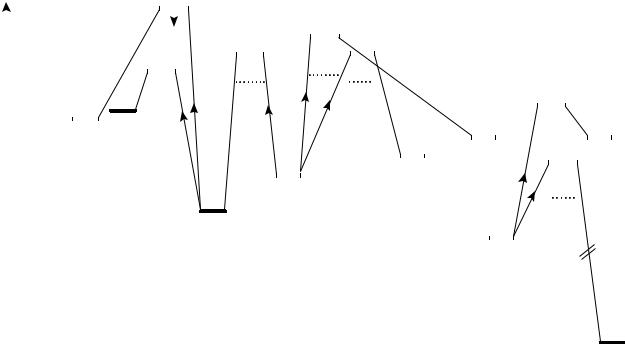
The complete potential energy surface of nitromethane, aci-nitromethane and methyl nitrite is accurately known by experiment (Figure 7)52. Thus, the aci-nitromethane radical cation, based on appearence energy measurements, has been found as the more stable by 6 kcal mol 152 .
The aci-nitromethane ions decompose by the loss of Hž , OHž and H2O15,50,52; see Scheme 2.
CH2 NO+ + OH
O |
+ |
|
|
|
|
CH2 N |
|
|
CH2 NO2 |
+ + H |
|
|
|||
|
OH |
|
||
|
|
|
HCNO+ |
+ H2 O |
|
SCHEME 2 |
|
||
It has been shown that isomerization of the nitromethane radical cation to the aci-form is the rate-determining step for the loss of OHž15,50,52 ; cf Figure 7. This is in contrast to the elimination of H2O, where the final step becomes rate determining52; cf Figure 7. A very large isotope effect apparently operates in the nitro-to-aci isomerization50,53. Thus, kH/kD has been determined to be in excess of 5050. Such a high value points to quantum mechanical tunneling, which consequently has been used as a basis for disclosing the reaction53. As a result of the significant isotope effect, the isomerization cannot be traced in the case of D3-nitromethane50,53. However, the process is immediately observed for the D0, D1, D2 isotopomers50. The loss of the hydrogen atom has the highest energy requirement and occurs apparently directly from the aci-form52.
|
|
1150 |
|
|
|
|
|
1121 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
1100 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1094 |
1083 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
1083 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
1069 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
1059 |
|
|
|
|
1064 |
1059 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
1050 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1043 |
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
1036 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
−1 |
|
|
|
1031 |
|
[H CNO]+. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
molkJ |
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
[H |
CONO]+ |
+ Η. |
||||||||||||||||||
|
|
.ΟΗ |
|
|
|
|
|
|
|
|
|
|
1014 |
|
|
|
|
|
|
||||||||||||||||||||||||
|
|
|
[HCNO]+ . |
2 |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1014 |
|
|
|
||||||||||||
|
|
|
|
Η |
Ο |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1001 |
|
[H2CONO] |
|
|
2 |
|
|
|
|
|
|||||||||||
|
|
1000 |
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+. |
|
|
|
995 |
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
987 |
|
|
|
|
|
|
[NO]+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
]+ . |
+ . |
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[H |
CNO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
3 |
2 |
|
|
|
|
H3CO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
958 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
965 |
|
|
|
|
|
|
|
|||||||||
|
|
950 |
|
|
|
|
|
|
|
|
|
[H2C |
= N(O)OH]+ . |
|
|
|
|
|
|
|
|
|
|
935 |
|
+ . |
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[H3CONO] |
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
900 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
794 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[CH2OH]+ |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ΝΟ+ . |
|
||
FIGURE 7. The potential energy surface of nitromethane, aci-nitromethane and methyl nitrite52. The dots indicate transitions determined from daughter ion appearance energies52. Such values may possess a significant kinetic shift3
261
262 |
Helge Egsgaard and Lars Carlsen |
2. N O isomerization: Nitro/nitrite radical cations
The N O isomerization may be visualized by the generation of NOC vs the loss of NO from the molecular ion. In the case of nitromethane, the formation of NOC may be unexpected because methyl nitrite ions decompose unimolecularly to CH2OHC and NO30,52,54 56; see Scheme 3. However, the isomerization gives rise to highly excited methyl nitrite ions, which will favour a fragmentation with a high frequency factor, i.e. a simple cleavage instead of the formal 1,2-hydrogen shift to an ˛-distonic ion. The latter has recently been suggested to be a common intermediate in the surprisingly complex unimolecular fragmentation of the methyl nitrite radical cation57.
NO+ + CH3 O
+ |
O |
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
||
CH3 N |
|
|
|
|
CH3 |
|
O |
|
NO |
|
|
O |
|
|
|
|
|||||||
|
|
|
|||||||||
|
|
|
|||||||||
|
|
||||||||||
CH2 OH+ + NO
SCHEME 3
Finally, it should be recalled that the neutral CH3O species associated with the formation of NOC from the nitromethane radical cation is purely the methoxy radical7.
In the case of nitroaromatics, the NO loss may in addition take place by a rearrangement involving the ortho position11. The determination of the associated kinetic energy release has played a central role in the elucidation of the mechanisms. Thus, the amount of kinetic energy released from a series of substituted nitrobenzenes is para > ortho > meta if the substituents are electron-donating, whereas the reverse order is noted in the case of electron-withdrawing substituents58. The observations were rationalized in terms of the stability of the product ion and the ring sizes of transition states of the reactions, i.e. threeor four-membered ring size58.
Studies applying the PEPICO technique have, on the other hand, lead to a much more refined picture of those reactions, because PEPICO makes possible the measurement of dissociation rates and kinetic energy release of ions with selected internal energies43. Thus, the formation of C6H5OC and NOC from nitrobenzene ions proceeds via rate-determining transition states. Near the threshold the formation of NOC is most probably preceded by a nitro nitrite isomerization, since no model seems able to account for the observed small kinetic energy release by a direct fragmentation through a tight TS with a large reverse activation energy43. Increasing the internal energy gives rise to increase in the average kinetic energy release beyond that predicted by a loose TS model, e.g. nitro nitrite isomerization. This implies that the nitrobenzene ion at higher internal energies no longer completely isomerizes and NOC is produced directly through a rate-determining TS relatively high in energy43.
3. Aliphatic nitro compounds: Cyclization reactions
More than three decades ago 1-nitropropane was studied by deuterium labelling59. It was shown that the molecular ions of 1-nitropropane can decompose via two channels following the 1,5-hydrogen shift, i.e. a -hydrogen shift from the terminal methyl group to
6. Mass spectrometry of nitro and nitroso compounds |
263 |
oxygen in the nitro group59. One channel corresponds to an elimination of ethylene giving rise to the aci-nitromethane ions, as disccussed above. The alternative route involves the loss of a hydroxyl radical. The resulting ions undergo a series of very specific rearrangements leading to loss of C3H5ž , H2O, HCN and CH2O, respectively51,59; see Scheme 4. Those processes have been studied by tandem mass spectrometry51.
+
CH2 |
|
|
CH2 |
|
|
CH2 |
|
|
NO2 |
|||
|
|
|
|
|
||||||||
|
|
|
|
|
|
− |
OH |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
CH2 |
|
|
CH2 |
+ |
|
||||
|
|
|
|
|
N |
O |
||||||
|
|
(1) |
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
H2 C |
|
H |
−C3 H5 |
+ |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
CH2 |
|
|
|
CH2 |
|
|
CH |
|
|
|
N |
|
|
|
O |
|
|
|
|
|
|
+ |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
(2) |
|
|
|
|
|
|
H |
|
|
|
|
|
H2 C |
C |
|
HNO |
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N O |
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
H2 C |
CH2 |
|
|
|
|
|
|
|
|
|
|
C2 H4 N+ + |
|
|
|
|
|
||||||||||||
|
|
|
|
|
O + |
|
CH |
|
|
|
|
|
|
|
|
|
|
CH2 O |
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
C H O+ |
+ |
HCN |
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
HO |
|
|
|
CH |
|
|
CH |
|
|
|
CH |
|
|
N |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
|
|
2 |
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
5 |
|
|
|
|
|||||||||
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C3 H4 N+ + H2 O |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
H2 O |
|
CH2 |
|
|
CH2 |
|
|
|
C |
|
N |
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
SCHEME 4
The proposal that ions of type 2 undergo ring closure to threeand five-membered rings whereas ions of type 1 do not cyclize to four-membered ring, fits nicely with the concept of -assisted/ -resisted cyclization reactions60.
Fragmentation of the nitroethylene radical cation has been studied by deuterium labelling and tandem mass spectrometry and compared to the fragmentation of the corresponding nitrosoethylene61.

264 |
|
Helge Egsgaard and Lars Carlsen |
|
|
|
H |
H |
H |
D |
D |
H |
H |
NO2 |
H |
NO2 |
D |
NO2 |
It appears that the radical cation of nitroethylene in the msec time frame rearranges to ionized nitrosoacetaldehyde via the 4H-oxazet N-oxide61. The informative part of the spectra corresponds to the ions originating from CHOž and H2CO losses from the molecular ion61.
|
H |
+ |
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
+ |
||
H2 C |
|
|
|
|
O |
|
N |
|
CH2 |
|
CHO |
||||
|
|
N |
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
||||||||||
|
NO2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|||||||||||
|
|
|
|
|
|||||||||||
D. Hydrogen Transfer Reactions: The ortho-Effect
One of the best known ortho-effects is the loss of a hydroxyl radical resulting from the interaction between a nitro group and a hydrogen atom in an adjacent substituent through a cyclic TS62.
+ |
+ |
X |
X |
H |
−OH |
O |
|
Y |
Y |
However, it should be emphasized that the OHž loss also occurs from meta- and para- substituted nitrobenzenes provided a side-chain of more than one carbon atom and at least one ˛-hydrogen atom is present. This loss of OHž shows up to a minor, but nevertheless significant, extent.
Mass spectrometry of 2-nitrotoluene, which may be regarded as a prototype in this context, was studied as early as 195963 and has since been studied intensively. By deuterium labelling it has been demonstated that the hydrogen lost through a OHž comes exclusively from the methyl group64. The dissociation dynamics of energy-selected 2-nitrotoluene radical cations has been studied by photoelectron photoion coincidence spectroscopy (PEPICO)65. The breakdown diagram and the OHž loss rates were determined. The measured rates were consistent with a mechanism in which the parent 2-nitrotoluene radical cation rearranges to an energetically more favourable structure prior to the loss of OHž . The rearranged ion was calculated to be 1.62 eV more stable than the 2-nitrotoluene radical cation and to disscociate with a critical energy of 1.5 eV65.
The kinetic energy release for the loss of OHž from the 2-nitrotoluene ion has been reported66. The relatively small value, 20 50 meV66, is characteristic of reactions involving a simple bond cleavage with neglectable reverse activation energy3. This implies that the final step, in a by-necessity complex reaction, is likely to be a simple bond cleavage.
The actual nature of the [M OHž ]C product ion has been approached by several research groups. Beynon and coworkers have discussed possible mechanisms for the
6. Mass spectrometry of nitro and nitroso compounds |
265 |
loss of OHž radical67. Thus, it has been suggested that the molecular ion undergoes rearangement to the nitrite form prior to the OHž loss (Scheme 5). Such a rearrangement is frequently invoked in the fragmentation of both aliphatic as well as aromatic compounds. In the second mechanism, no specific intermediate was claimed. Meyerson and coworkers, on the other hand, suggested that the fragmentation takes place straightforwardly via an intermediate formed by an initial simple hydrogen transfer, this product ion, hence, not containing a second ring system68.
|
O |
+ |
N |
|
|
||
|
|
H2 C |
|
CH3 N |
|
+ |
|
O |
|
|
O |
|
|
|
|
|
|
−OH |
|
|
|
+ |
|
+ |
|
O |
|
|
H2 C |
|
|
CH3 |
|
N |
|
NO2 |
|
|
|
−OH |
|
|
|
CH2 |
CH2 |
|
−OH |
+ |
OH |
N |
N+ |
O |
O |
|
SCHEME 5
A subsequent study of the unimolecular dissociations of a number of precursor molecules leads to the conclusion that the [M OHž ]C product ion possesses the 1,2-benzisoxazolenium structure, although the coexistence of other structures could not be ruled out67. This assignment is, however, partly in conflict with previous results demonstrating that the subsequent facile loss of CO from the [M OHž ]C product ion involves exclusively the carbon from the methyl group67,69. This inconsistency may be sought for in the uncertainty of the structures of some of the applied reference ions. Thus, the exact mechanism for the loss of OHž remains uncertain as does the actual identity of the product ion. The heat of formation, 900 š 15 kJ mol 1, predicted for [M OHž ]C , may turn out as highly diagnostic in establishing the structure in future experiments65.

266 |
Helge Egsgaard and Lars Carlsen |
The possibility that the loss of OHž from the molecular ion of ethylnitrobenzenes takes place from a common structure has been comprehensively studied64,70 73. It appears that the efficiency of the process is ortho > meta > para. By deuterium labelling, a high specificity for the involvement of the ˛-hydrogens in the loss of OHž from the ortho isomer has been demonstrated64,71. In contrast to this, the losses of OHž from the meta and para isomers appear much less favoured. In the latter cases the loss of OHž is preceded by an extensive hydrogen scrambling, leading to a virtual ‘loss of identity’71. The critical energy for the loss of OHž for the ortho isomer is remarkably low (0.05 eV) whereas the critical energies from the meta and para isomers are substantially higher, by approximately 1 eV71. In addition, the structures of [M OH]C ions have been studied by measurements of metastable ion spectra, collisional activation MS, kinetic energy release, photodissociation and critical energies for the formation of these ions and their subsequent decomposition70 73. It has been shown that for the ortho isomer the loss of OHž is a unique reaction and totally different from that of meta and para isomers; cf Figure 872. In addition, the rates of unimolecular decomposition of the metastable MCž and [M OH]C are different for meta and para isomers72. The collision-induced MS of [M OH]C show significant differences as well. On this basis, a common structure, e.g. a
FIGURE 8. Metastable ion spectra of [M OH]C ions of isomeric ethylnitrobenzenes71

6. Mass spectrometry of nitro and nitroso compounds |
267 |
methylnitrocycloheptatriene ion to be involved in the loss of OHž from ethylnitrobenzenes, was unambiguously rejected71.
1. Rearrangements due to ortho-effects
The loss of isobaric neutral species from the molecular ions of isomeric nitroanisoles has been studied using deuterium labelling74. The mass analysis indicated that specific loss of CH2O occurs from the molecular ions of 2-nitroanisole, while a specific loss of NO takes place from 3-nitroanisole74. Although the peak due to [M NO]C is neglectible in the MS of 2-nitroanisole, it is apparently an important transient intermediate in the consecutive fragmentation of the molecular ions.
The mechanism for loss of CH2O from 2-nitroanisole is highly unusual, as evidenced by the study of 2-nitro-18O-anisole and 4-nitro-18O-anisole75. The label of 4-nitro-18O- anisole is completely retained in the consecutive loss of NO and CO, as expected from an isolated nitro group. The fragmentation of 2-nitro-18O-anisole shows a loss of CH2O maintaining the 18O-label in the fragment ion [M CH2O]C , demonstrating that the oxygen atom of CH2O comes from the nitro group rather than from the methoxy group75. By contrast, the CO lost from the low-intensity [M NO]Cž ion contains the label, hence demonstrating that the methoxy oxygen is involved in the CO elimination in this case75. A rationalization of this complex mechanism has been presented75; see Scheme 6.
+ |
OH |
|
+ |
OH |
|
|
|||
|
|
N |
||
NO2 |
N |
|
|
|
|
+ |
O |
−CH2 O |
|
|
|
|
||
|
18 O |
CH2 |
|
|
18 OCH |
|
|
18 O |
|
3 |
|
|
|
|
|
SCHEME 6 |
|
|
|
The characterization of the [M NO CO]C ions, i.e. C5H5OC of o-, m- and p- nitrophenol, has been attempted based on translational energy release measurements on metastable ions76. It appeared that in all cases the pyrylium structures closely resemble that observed for the high-energy ions, i.e. when fragmentation of the C5H5OC ions takes place in ion source. However, when the slower reactions occurred in a field-free region the values for kinetic release did not coincide with any of the structures investigated. Thus, it seems clear that at least one additional structure has to be included to account for the fragmentation of the long-lived ions76; see Scheme 7.
OH +
NO2
− NO − CO
O
+
SCHEME 7
268 |
Helge Egsgaard and Lars Carlsen |
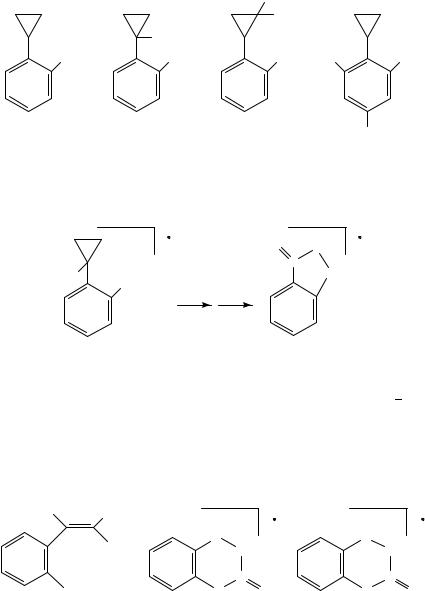
The alkene loss from ionized cycloalkyl-substituted nitrobenzenes has been studied by isotopic labelling and collision activation mass spectrometry77. The reaction path was found to depend highly on the placement of the nitro group. The ortho nitro-substituted phenylcyclopropane and its isotopomers were studied.
D
D
D
NO2 |
NO2 |
NO2 |
D |
NO2 |
D
It appeared that, exclusively, the methylene hydrogens of the cyclopropane are involved in the ethylene formation77. The product ion, [M C2H4]Cž , was by collision activation MS found to be perfectly identical with that of the molecular ion of [2,1]-benzisoxazoline- 3-one77; see Scheme 8.
|
+ |
O |
+ |
|
|
O |
|
|
|
|
|
|
|
|
C |
H |
NO2 |
|
NH |
|
|
||
|
|
|
|
|
|
− C2 H4 |
|
SCHEME 8
The corresponding para nitro-substituted cyclopropane does not eliminate ethylene. However, alkene elimination is observed for the higher homologues, i.e. n D 3 5. The product ion has in this case been proved to be 4-nitrostyrene77; see Scheme 9.
Another classical case with respect to ortho-effects is found for 2-nitrostyrene78. The conceivable regioand stereo-specifically labelled 2-nitrostyrenes have, in addition to the ring-labelled isotopomer, been studied by collision activation mass spectrometry79. Undoubtedly, the most striking result was the nearly equal contribution of both (in the neutral molecule diastereotopic) hydrogens of the ˇ-carbon.
Hα |
Hβ |
H |
+ |
H2 |
+ |
|
|
||||
|
|
|
|
||
|
Hγ |
C |
|
C |
|
|
|
CH2 |
|
CH |
|
|
|
|
|
||
|
|
|
N |
|
N |
NO2 |
|
O |
O |
O |
O |
|
|
(3) |
|
(4) |
|
