
6. Mass spectrometry of nitro and nitroso compounds |
269 |
+
CnH2 n
+
H CH2
−CmH2 m
NO2 |
NO2 |
SCHEME 9
The collision-induced spectra of the [M OD]C ions generated from the regioand stereo-specifically labelled compounds revealed identical MS, suggesting the formation of a common ion structure for this long-lived daughter ion79. A tentative rationalization was based on an initial isomerization, leading to the radical cation of the corresponding nitrite followed by an electrocyclic ring closure to yield the nitronic ester 379. Hydrogen exchanges may take place consecutively by facile 1,2-hydrogen shifts within the structures 3 and 4. The eventual loss of OHž was assumed to take place following ring-opening of the nitronic ester functionality and a subsequent rearrangement of the C-nitroso compound to the corresponding oxime79.
The electron impact mass spectra of 3-methyl-4-nitro-5-styryl-isoxazoles exhibit, on the contrary, only negligible loss of OHž80 . This has been interpreted in terms of an isoxazole- to-azirine rearrangement80. The latter fragments directly to an abundant cinnamoyl ion as well as rearranges to oxazole and an epoxide through an intramolecular oxidation of the ethylenic bond by the nitro group80; see Scheme 10.
|
+ |
+ |
|
NO2 |
CH3 |
CH3 |
|
|
|||
H |
|
NO2 |
|
C CH |
N |
||
N |
|||
Ar |
O |
Ar CH CH C |
|
|
|
O |
|
|
|
SCHEME 10 |
2. ortho-Effects in anions
It is interesting to note the effect of charge on hydroxyl loss from ortho-substituted nitrobenzene ions. The loss of OHž is observed from many ortho-substituted nitrobenzene molecular anions66. This ortho-effect is often operative in apparent analogy with the corresponding cations. The results can be rationalized if an intramolecular proton transfer is
270 |
Helge Egsgaard and Lars Carlsen |
assumed as a first step as opposed to an intramolecular hydrogen transfer in the corresponding cations66. This assumption is further supported by the low, less than 50 meV, kinetic energy release, which obviously is consistent with a simple cleavage with a low reverse critical energy as second step; see Scheme 11.
O |
O− |
O |
H |
|
−OH |
|
|
|
O− |
|
OH |
N |
N |
N |
|
|
O− |
O |
O |
|
SCHEME 11
As a consequence, a higher degree of charge localized at the substitutents in the transition state for proton transfer in the anions is developed. Thus, the relative importance of the OHž loss is directly related to the capability of the substituent to accept a negative charge, and hence as reflected in the gas-phase acidity of the parent compound66. In contrast, very little charge is localized at the reaction site in the transition state for hydrogen transfer within the cations. Thus, in this case, the relative importance of the OHž loss seems to correlate to the homolytic bond strength which has to be overcome66.
E. Remote Oxidation
1. Remote oxidation of multiple carbon bonds
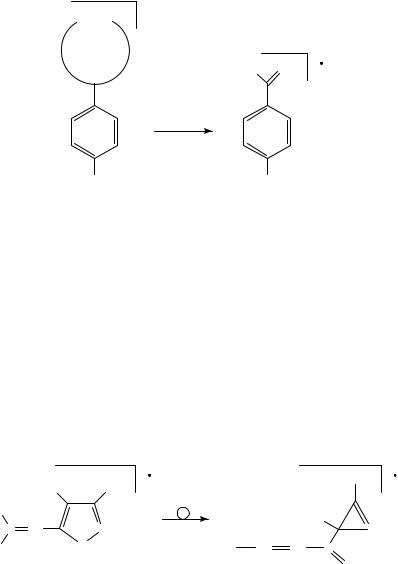
The oxygen transfer from an ortho nitro group to a carbon carbon triple bond has been studied by high-resolution mass spectrometry, linked scan techniques and chemical substitution81. Oxygen transfers to both acetylenic carbons were detected as parallel fragmentation pathways81.
α β
C C
NO2
The oxygen transfer to the ˇ-acetylenic carbon results in the very intense benzoyl cation, whereas the transfer to the ˛-carbon, via a series of fragments corresponding to the loss of OHž , CO and CO2, respectively, leads to annelated heterocycles such as the radical cation of carbazole, as a result of elimination of CO81. The generation of the benzoyl cation was rationalized as shown in Scheme 1281.
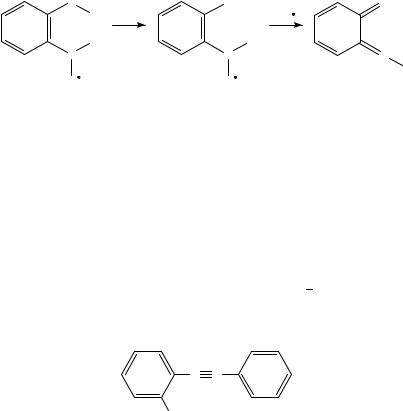
Oxygen transfers from the nitro group to allenic double bonds have been studied in 2-nitrophenyl allenylmethyl ether82. The ortho interaction is obviously in agreement with the observed elimination of both the C4H5Ož radical and C4H4O from the molecular ion82; see Scheme 13. The loss of C4H4O is followed by elimination of a hydrogen atom. Thus, the two routes apparently result in a common fragment ion82. The [M C4H4O]Cž ion is also observed in the study of the para isomer. However, in the latter case the fragment ion is formed by a completely different pathway, i.e. by the subsequent losses of C4H4 and atomic oxygen, respectively82.

|
6. Mass spectrometry of nitro and nitroso compounds |
271 |
|||
Ph |
|
Ph |
|
Ph |
+ |
|
|
|
|||
|
|
|
|
|
|
C |
|
C |
O |
O C |
|
|
|
|
|
|
|
C |
O |
+ C |
|
O |
O |
|
|
||||
|
N+ |
|
|
N |
N |
|
|
|
|
||
O
O
+ C
|
|
SCHEME 12 |
|
O CH2 |
+ |
+ |
|
|
|
O CH2 |
|
CH |
|
|
CH |
C |
|
|
O C |
NO2 |
CH2 |
|
NO |
|
|
CH2 |
|
|
|
|
− C4 H4 O + H / C4 H5O |
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
+ |
|
|
|
|
N |
|
|
|
O |
SCHEME 13
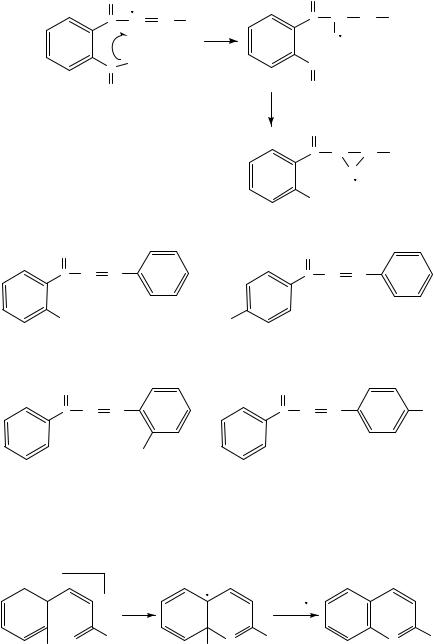
The electron impact and chemical ionization mass spectra of selected nitrosubstituted isomeric benzalacetophenones, benzyl ketones and aromatic epoxides have been examined83. The isomeric pairs display rather significant differences following ionization. Thus, the ortho nitrobenzalacetophenone 5 shows an abundant series of ions due to fragmentation of the epoxide intermediate originating from an oxygen transfer from the nitro group. The first step is an abstraction of the oxygen by the radical site located on the ˇ-carbon. These processes are virtually absent in the para isomers 6 and 883. The fragmentation of the para isomers presumably leads to a series of cyclized product ions83; see Scheme 14.
272 |
|
|
Helge Egsgaard and Lars Carlsen |
|
|
|
||
|
|
O |
|
|
O |
|
+ |
|
|
|
+ |
|
|
|
|
||
|
|
|
|
C |
CH |
Ph |
||
|
|
C CH CH |
Ph |
CH |
||||
|
|
|
|
|
|
|||
|
|
|
|
|
|
O |
|
|
|
|
N |
O |
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
C |
CH |
CH |
Ph |
|
|
|
|
|
|
|
+O |
|
|
|
|
|
|
NO |
|
|
|
|
|
|
|
SCHEME 14 |
|
|
|
|
O |
|
|
|
|
O |
|
|
|
C |
CH |
CH |
|
|
C |
CH |
CH |
|
NO2 |
|
|
|
NO2 |
|
|
|
|
|
(5) |
|
|
|
(6) |
|
||
O |
|
|
|
|
O |
|
|
|
C |
CH |
CH |
|
C CH |
CH |
|
NO2 |
|
|
|
|
NO2 |
|
|
|
|
|
|
(7) |
|
|
|
(8) |
|
|
|
A comparison of the spectra of the ortho isomers, 5 with 7, reveals that fragmentation due to oxidation of the styryl double bond apparently is absent in 7. Instead, the MS of the latter exhibits ions corresponding to the formation of a benzopyrylium ion; see Scheme 15.
+ 
|
|
|
|
−NO2 |
|
O |
Ph |
+ |
Ph |
+ |
Ph |
O |
O |
||||
O2 N |
|
NO2 |
|
|
|
SCHEME 15
6. Mass spectrometry of nitro and nitroso compounds |
273 |
The MS of the epoxides 9 and 10 appear as rather interesting, since they may be regarded as the nitro analogues of the nitroso-epoxide intermediates discussed above.
O |
O |
|
O |
O |
C |
CH CH |
|
C |
CH CH |
|
|
NO2 |
NO2 |
|
|
|
|
|
|
|
(9) |
|
|
(10) |
The cleavage ˛ to the carbonyl (and the epoxide) functionality generates the acylium ions in high yield. Thus, the benzoyl cation is the base peak in the mass spectrum of 983. However, the nitro group ortho to the carbonyl group in the isomer 10 significantly influences the fragmentation due to the interaction with the acylium group, the latter formally being a result of the ˛-cleavage. A subsequent loss of atomic oxygen accounts for the most abundant ion in this spectrum83; see Scheme 16.
+ |
|
|
|
O |
O |
|
O |
|
|
|
|
C CH CH Ph |
|
C |
|
|
|
−PhC2 H2 O |
|
|
|
|
O |
|
|
|
|
|
|
+ |
|
NO2 |
|
|
N |
O
−O
O
C
+
O
N
SCHEME 16
The observed differences in the fragmentation pathways of the isomeric pairs were further enhanced under chemical ionization conditions. Thus, elimination reactions became even more pronounced in the ortho-nitro-substituted compounds than in the para isomers83.
2. Remote oxidation of imine and ketenimine functionalities
The mass spectra of heterocycles containing an integrated 2-nitrobenzaldehyde-imine moiety exhibit a m/z 134 ion of significant abundance84. The study of m/z 134 was based on MIKE spectra supplementary to measurements of kinetic energy release84. Accordingly, the m/z 134 was assigned to the o-quinoid structure 11, the latter being proposed to be generated by isomerization of the precursor ion to give a spiro intermediate from

274 |
Helge Egsgaard and Lars Carlsen |
which the m/z 134 arises synchronously. The mechanism represents an oxygen transfer from the ortho nitro group to the imino carbon via a five-membered transition state84; see Scheme 17.
+ |
+ |
Z
Y Z
Y
X N O N O X N O N O
O
C
+
N
O
(11)
SCHEME 17
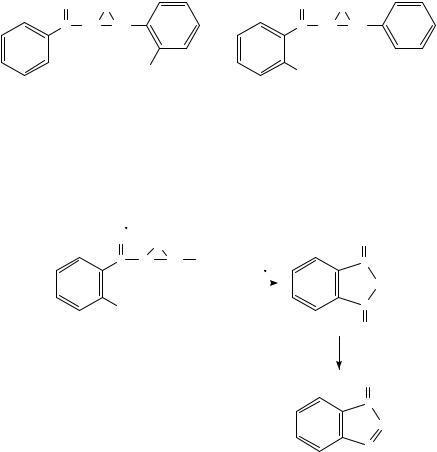
Illustrative examples of intramolecular oxidations of remote groups in nitroaromatic ions are the redox reactions occurring in ionized benzotriazoles and triazolopyridines bearing o-nitroaryl substituents on nitrogen85. Dissociative ionization apparently causes
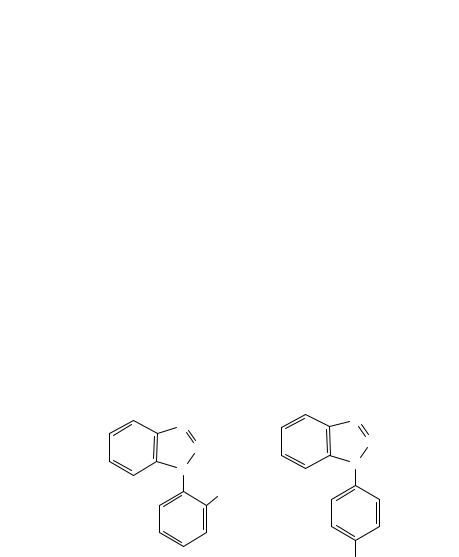
(a)
FIGURE 9. Electron impact mass spectra of (a) 1-(2-nitrophenyl)benzotriazole, 12, and (b) 1-(4- nitrophenyl)benzotriazole, 13, respectively85. Reprinted from Reference 85 with kind permission of Elsevier Science-NL, Sara Burgerhartstraat 25, 1055 KV Amsterdam, The Netherlands
6. Mass spectrometry of nitro and nitroso compounds |
275 |
(b)
FIGURE 9. (continued)
an intramolecular process involving the o-nitro group and the benzene ring of the annelated triazole. The importance of the ortho position of the nitro group is unambiguously demonstrated by the MS of 1-(2-nitrophenyl)benzotriazole 12 and 1-(4-nitrophenyl)benzotriazole 13 given in Figure 985. The mass spectrum of 12 is very similar in the high-mass region to that of 13, but, on the other hand, very different in the low-mass region due to the base-peak (m/z 92) and its daughter ions.
N N
N N
N N
NO2
NO2
(12) |
(13) |
The significant behaviour of the compound 12 on electron impact ionization, i.e. the formation of a dominating ionic species, m/z 92, 14, has been rationalized as shown in scheme 1885. It should be noted that the intermediacy of the ionized iminocarbene was proposed along with the possibility that an oxygen transfer takes place directly from the nitro group to the annelated benzene ring. Similarly, ionized 2- and 3-azafulven-6-ones were demonstrated to be formed from the appropriate heterocycles85.

276 |
Helge Egsgaard and Lars Carlsen |
|
|
+ |
+ |
|
N |
|
|
N |
|
|
N |
N |
|
NO2 |
NO2 |
C N |
+ |
C |
O |
O |
+ |
|
|
|
N |
|
(14) |
|
O |
|
SCHEME 18 |
3. Competing oxidation of sulphur and carbon
Competing oxygen transfers from ortho nitro groups to sulphur and carbon, respectively, have been studied for allyl sulphide, styryl sulphides, allenyl sulphides and ethynyl sulphides86 89.
Oxygen transfer from the nitro group to the CDC group in allyl 2-nitrophenyl sulphide followed by a simple cleavage resulted in intense fragment ions corresponding to ionized 2-nitrosothiophenol and the related thioquinoid structure, respectively86; see Scheme 19. Further, a double oxygen transfer from the nitro group to the sulphur atom has been suggested in order to account for the extrusion of HSO2ž from the molecular ion86; see Scheme 20. By collision activation the product ion was disclosed as protonated quinoline86.
The allyl 2-nitrophenyl sulphoxide is apparently not an intermediate in this reaction as the MS of the latter compound is dominated by a cleavage reaction giving rise to C3H5C , whereas the ions corresponding to a loss of the HSO2ž radical apparently are of low intensity86. Thus, it was concluded that the double oxygen transfer to sulphur most probably should be formulated as a concerted reaction86.
The double oxygen transfer to sulphur is also a characteristic feature of 2-nitrophenyl styryl sulphides87. The rearranged molecular ion undergoes fragmentation in two parallel pathways. Elimination of SO2 affords the radical cation of 2-phenylbenzopyrole and is followed by a loss of Hž ; see Scheme 21. In addition, this ion is formed by a direct loss of HSO2ž from the rearranged molecular ion87.
The additional double bond in the corresponding allenyl 2-nitrophenyl sulphides causes a significant preference of the oxygen transfer to carbon88. The dominant ions correspond to the radical cations of 2[3H]-benzothiazolone and protonated benzothiazole, respectively88. Both ions require the transfer of two oxygens to carbon in the side-chain as well as extensive rearrangements of the molecules; see Scheme 22. The transfer of
6. Mass spectrometry of nitro and nitroso compounds |
277 |
||
+ |
|
S |
+ |
S |
|
|
|
|
|
O |
|
NO2 |
|
NO |
|
− C3 H4 O |
|
|
|
|
|
− C3 H5O |
|
+ |
|
|
S |
SH |
|
|
|
|
|
|
+ |
NO |
|
|
N |
|
|
|
O |
SCHEME 19 |
|
|
|
+ |
O |
O |
+ |
S |
|
S |
|
|
|
|
|
NO2 |
|
N |
|
|
|
|
|
|
|
−HSO2 |
|
+
N H
SCHEME 20
oxygen to sulphur is, on the other hand, visualized through a minor loss of SO from the molecular ion88. It should be noted that the proposed ortho interactions in the allenyl 2-nitrophenyl sulphide are virtually absent in the spectrum of the corresponding para isomer88.
It seems interesting to compare the mass spectrometric features of 2-nitrophenyl- phenylethynyl sulphides with the fragmentation of the nitrodiphenylacetylenes discussed above. Apparently, oxygen transfers to both the acetylenic carbons and sulphur take place89. The transfer of a single oxygen to the ˇ-carbon leads, via a mechanism closely resembling the fragmentation of nitrodiphenylacetylenes, to the benzoyl cation89. The latter ion apparently is the base peak in the MS of the major part of the compounds studied89; see Scheme 23.
278 |
Helge Egsgaard and Lars Carlsen |
|
|
|
H |
Ph |
+ |
|
+ |
|
O |
O |
||
|
|
|
||
C |
C |
|
|
S |
|
|
|
||
S |
H |
|
|
|
NO2
S
CH:C
NO2
+
S CH
O
N
H
|
N |
|
Ph |
|
H |
|
|
|
−SO2 |
|
|
|
|
|
+ |
|
|
|
Ph |
|
N |
|
|
|
H |
|
|
|
SCHEME 21 |
|
|
|
+ |
|
|
|
S C C |
CH2 |
|
|
O |
|
|
|
O |
|
|
|
N |
|
|
|
− C2 H2 O |
|
|
|
H |
|
|
+ |
|
S |
+ |
CH2 |
|
|
|
|
|
C O |
|
|
|
|
|
|
|
N |
|
|
|
H |
|
C CH |
|
|
S |
|
|
|
|
O |
−[CO + CHO] |
|
CH |
|
|
||
|
|
|
+ |
|
|
|
N |
|
|
|
H |
SCHEME 22
