
6. Mass spectrometry of nitro and nitroso compounds |
|
279 |
|
|
S |
Ph |
+ |
|
|
||
+ |
|
|
|
C |
C |
|
|
S C C Ph |
|
O |
|
|
|
|
|
NO
NO2
|
O |
|
S |
C |
|
|
+ |
|
C |
Ph |
|
O+ |
Ph |
C O |
|
|
|
N |
|
|
SCHEME 23
The transfer of two oxygens to the acetylenic carbons involving a series of rearrangements, followed by the loss of two CO molecules, leads to the phenothiazine radical cation, with the proposed fragmentation pathway being supported by collision activation MS89; see Scheme 24. Although the transfer of oxygen to sulphur apparently is of minor importance, the loss of a HSO2ž radical from a rearranged molecular ion seems to resemble the mechanism proposed for the equivalent fragmentation of allyl 2-nitrophenyl sulphide and 2-nitrophenyl styryl sulphides86,87,89.
|
|
S |
Ph |
+ |
|
|
|
||
|
|
|
|
|
|
+ |
|
C C |
|
S C C Ph |
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
NO |
|
|
NO2
|
|
|
O |
+ |
|
|
O |
S C |
|
|
|
|
O |
|
S |
|
C |
|
|
|
C |
|
||
|
C |
|
|
|
|
|
|
|
|
|
O |
+ |
|
|
N |
|
NH |
|
|
|
|
|
||
H |
|
|
−2 CO |
|
|
|
|
|
|
|
|
S |
+ |
|
N
H
SCHEME 24
280 |
|
Helge Egsgaard and Lars Carlsen |
Since |
the |
nitro group apparently is able to transfer oxygen atoms to substituents |
in the |
ortho |
position containing multiple bonds or heteroatoms, the N-arylidene- |
2-nitrobenzenesulphenamides constitute an interesting class of compounds90. Thus, unexpected ortho interactions of the nitro groups apparently operate as the molecular
ions expel SO2 and N2, |
leading to |
a rather abundant hydrocarbonic |
ion |
assigned |
||
as 1,2-phenylenetropylium90; see Scheme 25. The loss of HSO2ž |
is |
of |
minor |
|||
significance90. |
|
|
|
|
|
|
|
|
O |
O |
|
|
|
S N |
CH Ph |
+ |
S |
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
CH |
Ph |
|
|
|
|
N |
|||
NO2 |
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
−SO2 |
|
|
|
|
|
|
−N2 |
|
|
|
|
|
|
−H |
|
|
|
|
SCHEME 25
A competing oxygen migration in ortho nitroaryl thioamides on electron impact has been reported91. The loss of SO from the molecular ions is a highly significant process91. A mechanism involving an initial oxygen migration to the sulphur atom through a favourable six-membered TS followed by cyclization with concomitant expulsion of SO has been proposed91; see Scheme 26.
1 |
2 |
O |
|
R1 |
R2 + |
R |
R |
|
|
||
N |
|
S |
|
|
N |
|
|
|
|
|
|
C |
S |
C + |
1 2 |
|
C |
|
NR R |
−SO |
|
||
|
|
|
|
O |
|
|
|
|
|
|
|
+ |
O |
NO |
|
|
N |
N |
|
|
|
||
|
|
|
|
||
O
SCHEME 26
The migration of an oxygen atom from the nitrogen of the nitro group to the nitrogen moiety of the thioamide results in the formation of a stable o-nitrosothiobenzoyl cation91. It is assumed that the actual mechanism to be initiated by the migration of oxygen through a six-membered transition state is followed by an ˛-fission91; see Scheme 27.
6. Mass spectrometry of nitro and nitroso compounds |
281 |
||
S |
|
S |
|
C |
R1 |
C + |
R1 |
|
|
||
N |
R2 |
N |
R2 |
|
|
||
+ O |
|
O |
|
N |
|
NO |
|
O |
|
− ONR1R2 |
|
|
|
||
|
|
+ |
S |
|
|
||
|
|
C |
|
NO
SCHEME 27
F. Polynitro Aromatics
1. Nitroadamantanes
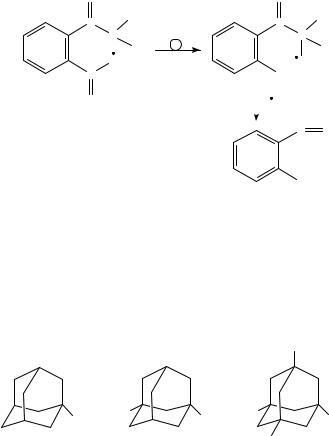
The fragmentation pathways of adamantane, 1-nitroadamantane, 1,3-dinitroadamantane and 1,3,5,7-tetranitroadamantane have been studied by tandem high-resolution MS92. It was found that the fragmentation of the nitroadamantanes was initiated by consecutive losses of the nitro groups and followed by fragmentation of the hydrocarbon back-bone92.
|
|
|
NO2 |
O2 N |
NO2 |
O2 N |
NO2 |
NO2 |
|
||
|
|
NO2 |
|
2. Diand tri-nitroaromatics
The fragmentations of 2,4,6-trinitrotoluene have been studied using isotope labelling and MS/MS techniques93,94. The major pathways include the loss of OHž and H2O, followed by the subsequent loss of NO or NO2. The facile transfer of a methyl hydrogen to oxygen gives rise to the key fragment (m/z 210) initiating a number of important fragmentation sequences93. There is virtually no ring disintegration until the majority of the attached groups are lost94.
In addition, a series of dinitroaromatic compounds has been studied by collision activation MS95. Strong effects due to competitive processes were noted95.
G. Nitro-heteroaromatics
1. Nitroazoles
The substituted heteroaromatic compounds provide excellent opportunities for mechanistic studies of the interaction between the nitro group and other substituents. This is due

282 Helge Egsgaard and Lars Carlsen
to the unique synthetic possibilities as well as a more straightforward evaluation of the role of the aromatic back-bone. Thus, MS of nitroazoles, i.e. nitropyrazoles and nitroimidazoles, has been investigated in detail by the effect of substitution and isotope labelling by Luijten and Thuij96 99.
Various nitropyrazoles and nitroimidazoles have been studied96 99; see Table 1. The loss of OHž from methyl-substituted nitropyrazoles and nitroimidazoles appears as a useful probe in determining the actual location of the substituents. Thus, it could be established that in the case of adjacent methyl and nitro groups, the loss of OHž originates exclusively from these substituents96 98. In addition to the loss of OHž the presence of a mobile N-bonded hydrogen atom leads to the elimination of H2O, as is visualized by the mechanisms for loss of OHž and H2O from 3(5)-methyl-4-nitropyrazole and 4-methyl- 3(5)-nitropyrazole, respectively98; see Scheme 28.
|
+ |
O |
|
H |
+ |
OH |
|
|
|
|
O |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
CH2 |
|
CH2 |
|
|
|
|
+ |
|
||||||
O |
N |
|
O N |
|
|
|
|
|
CH2 |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
−OH |
|
|
|
|
|
|
|
|
NH |
|
|
NH |
|
|
|
|
|
NH |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
N |
|
|
|
|
N |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H2 C |
H |
O + |
H2 C |
O + |
|
|
|
|
H2 C |
|
|||||||
|
|
N O |
N OH |
|
|
|
|
NO |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
−H2 O |
|
|
||
|
|
|
|
NH |
|
|
N H |
|
|
|
|
|
N |
||||
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
N |
|
|
|
|
|
|
|
N |
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
SCHEME 28 |
|
|
|
|
|
|
|||
TABLE 1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R4 |
R3 |
|
|
|
|
|
R4 |
R3 |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
N |
|
|
|
|
|
R5 |
N |
|
|||||
|
|
R5 |
|
|
|
|
|
|
N |
|
|||||||
|
|
|
N |
|
|
|
|
|
|
|
|
R1 |
|
||||
|
|
|
|
R1 |
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
Pyrazoles |
|
|
|
|
|
Imidazoles |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||
R1 |
|
R3 |
|
R4 |
R5 |
|
|
R1 |
|
R2 |
R4 |
R5 |
|||||
H |
|
Me |
|
NO2 |
H |
|
H |
|
H |
Me |
NO2 |
||||||
H |
|
Me |
|
H |
NO2 |
|
H |
|
Me |
NO2 |
H |
||||||
H |
|
NO2 |
|
H |
H |
|
H |
|
NO2 |
Me |
H |
||||||
H |
|
NO2 |
|
Me |
H |
|
H |
|
NO2 |
H |
H |
||||||
Me |
|
H |
|
H |
NO2 |
|
Me |
|
H |
H |
NO2 |
||||||
Me |
|
H |
|
NO2 |
H |
|
Me |
|
H |
NO2 |
H |
||||||
Me |
|
H |
|
H |
NO2 |
|
Me |
|
NO2 |
H |
H |
||||||
Me |
|
NO2 |
|
H |
H |
|
|
|
|
|
|
|
|
||||

6. Mass spectrometry of nitro and nitroso compounds |
283 |
The presence of a significant kinetic isotope effect for the losses of OHž from the nitroazoles (kH/kD D 4.6) supports the suggestion that the fragmentations are adequately described by a stepwise hydrogen transfer, followed by cleavage of the OH moiety or, alternatively, rearrangement to structures which can eliminate OHž and H2O98.
Loss of small O-containing molecules, such as CHOž and CH2O, can also take place. Three structural requirements for these reactions have been identified: (1) The methyl and nitro group must be in a mutual ortho position. Further, the reaction is strongly favoured if (2) the methyl group is bonded to a ring nitrogen and (3) a second heterocyclic moiety or a second heteroatom is close to the methyl group99. This is reflected in the relative intensities of the fragment ions. The reaction mechanisms for loss of the formyl radical and the elimination of formaldehyde are illustrated by the fragmentations of 1-methyl-5- nitroimidazole and 1-methyl-2-nitroimidazole, respectively99; see Scheme 29.
|
|
N |
|
|
|
N |
|
N |
O |
+ |
|
HO + |
|
HO + |
|
||
|
N |
N |
|
N |
|
N |
N |
N |
|
|
|
|
|||||
|
|
|
O |
|
|
|||
|
O |
CH2 |
|
|
CH2 |
O |
CH2 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|||
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
N |
|
|
|
|
HO |
|
+ |
−CHO |
|
|
|
|
|
N |
N |
+ |
||
|
|
|
|
|
||||
|
|
|
|
|
O |
N |
|
N |
|
|
|
|
|
C H |
OH |
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
N |
|
|
|
N |
|
N |
|
|
|
+ O |
|
|
|
O |
−CH2 O |
|
|
|
|
+ |
|
N |
+ |
N O |
|
|
N |
N |
|
|
||||
|
|
|
N |
|
O |
N |
|
|
|
|
O |
H |
|
CH2 |
H |
|
|
H |
CH2 |
|
|
|
||||
|
|
|
|
|||||
|
|
|
|
|
|
|||
SCHEME 29
It should be noted that the operation of these effects is attributed to the interaction of substituents only. Thus, in no cases has involvement of ring atoms of the diazole in the losses of CHOž and CH2O been detected99. The use of the labelled compounds unambiguously demonstrated that the diazole ring of the molecular ion structures is retained in the fragment ion discussed above99.
The MS of some nitro derivatives of imidazole-4(5)-carboxaldehyde have been studied100. Based on the compounds 15 17, several new features caused by the interaction between the nitro group and adjacent substitutents were disclosed. It should be noted that for the compounds 16 and 17, there is a rapid equilibrium between the two possible tautomers resulting in equivalence of the 4 and 5 positions.
Surprisingly, the [M H2O]Cž and [M CH2O]Cž ions are observed, whereas there is virtually no loss of CHOž100 . The fragmentation pattern of 5(4)-nitro-imidazole-4(5)
284 |
Helge Egsgaard and Lars Carlsen |
||
|
OHC |
NO2 |
OHC |
|
N |
N |
N |
|
NO2 |
N |
NO2 |
|
N |
N |
|
|
CH3 |
H |
H |
|
|
|
|
|
(15) |
(16) |
(17) |
carboaldehyde, 17, corroborates the presence of the formyl group. Thus, the primary losses of OHž and H2O are consistent with the H-shift from the formyl group to the nitro group followed by the loss of OHž or by elimination of H2O via mechanisms similar to that reported for methylnitroimidazoles; see Scheme 30. The ion corresponding to loss of H2O is the base peak in the MS of 17100.
O |
|
O |
|
|
|
|
C |
|
|
|
O |
|
|
|
|
|
|
C |
|
|
N |
H |
C |
|
|
|
|
|
|
|
|
|||
|
N |
|
|
N |
||
|
|
|
|
|
||
+ |
|
|
|
|
|
|
O + |
|
|
HO + |
|
||
O N |
|
|
|
|||
N |
N |
N |
|
|
N |
N |
O |
|
|
|
|
||
|
|
|
|
H |
||
|
H |
|
|
O |
||
H |
O |
|
|
|||
|
|
|
||||
H |
|
|
|
|
|
|
−H2 O |
|
|
|
|
−OH |
|
|
|
|
|
|
|
|
O |
|
|
|
|
O |
|
|
|
|
|
C |
|
|
C |
+ |
|
|
|
|
|
|
|
|
|
N |
||
N |
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
ON |
|
|
|
O |
N |
N |
|
|
|
|
|||
|
|
|
|
|
||
N |
|
|
|
|
|
H |
SCHEME 30
The elimination of OHž and H2O has been explained by assuming an initial H-transfer from the formyl group to the nitro group, followed by a migration of a hydrogen atom from the methyl group to the methyl-carrying nitrogen atom in line with the fragmentation of ‘o-methylnitroimidazoles’100.
2. 3-Nitro-2H-chromenes and 3-nitrochromanes
The electron impact mass spectra of series of 2-aryl-3-nitro-2H-chromenes and 2-aryl- 3-nitrochromanes with varying functionalities have been reported101. The molecular ion is always of considerable intensity. Loss of NO2 from MCž is responsible for the base peak in the nitrochromenes101. In contrast, the [M HONO]Cž is apparently the more abundant ion in the nitrochromanes. This unusual loss of HONO is always observed in the metastable time-frame101.
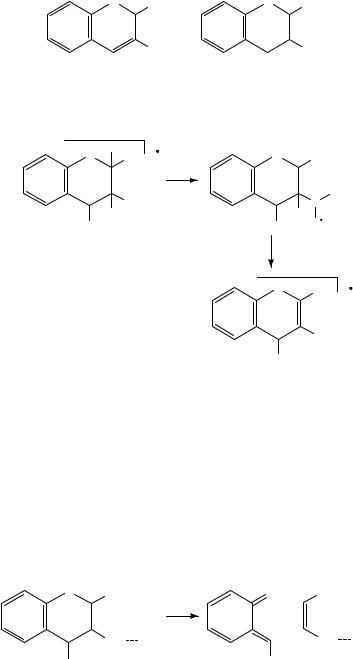
6. Mass spectrometry of nitro and nitroso compounds |
285 |
|||
O |
Ar |
O |
Ar |
|
|
NO2 |
|
NO2 |
|
The formation of [M HONO]Cž has been demonstrated by deuterium labelling to be associated with hydrogen transfer from the 2-position to the nitro group101. This leads to a stabilized radical cation which, following the expulsion of HONO, produces a very stable daughter ion; see Scheme 31.
O |
Ph |
+ |
O + |
|
|
H |
|
Ph |
|
||
|
NO2 |
|
|
N |
OH |
|
|
D |
|
||
|
D |
|
O |
|
|
D |
|
|
D |
|
|
|
|
|
−HONO |
|
|
|
|
|
O |
Ph |
+ |
|
|
|
|
|
|
|
|
|
|
D |
|
|
|
|
D |
|
|
SCHEME 31
All the compounds studied exhibited M ž accompanied by intense [M NO2] ž fragment ions in their electron capture negative ion chemical ionization spectra. Additional ions were of low intensity101.
Chemical ionization MS of 2-aryl-3-nitro-2H-chromenes and 4-hydroxy-3-nitroflavans have been studied using methane and ammonia as reagent gases102. The behaviour of 2-aryl-3-nitro-2H-chromenes was found to resemble that of aromatic nitro compounds. Thus, the methane spectra are characterized by the MHC ions, whereas the ammonia
spectra reveal dominant cluster ions, e.g. [M C NH4]C102 . Chemical ionization of the 4- hydroxy-3-nitroflavans leads, on the other hand, to a significantly increased fragmentation. Thus, the base peaks in the methane spectra are due to loss of H2OCNO2. In the ammonia spectra a rather complicated picture developed. Apparently, the dominant fragmentation is a retro Diels-Alder reaction of the ammonium adduct102; see Scheme 32.
O |
Ar |
O |
Ar |
|
|
|
+ |
|
+ |
|
+ |
|
NO2 NH4 |
|
NO2 NH4 |
OH |
|
OH |
|
SCHEME 32

286 |
Helge Egsgaard and Lars Carlsen |
H. Annelation Processes
Electron impact induced fragmentation of 2-nitrodiarylamines has been studied. An important path is the annelation to carbazoles, as illustrated by the fragmentation of 2- phenylamino-3-nitropyridine103; see Scheme 33. The identity of the product ions was confirmed by collision activation MS upon comparison with authentic samples103.
|
+ |
|
+ |
|
NO2 |
|
|
N |
N |
N |
N |
|
H |
|
H |
SCHEME 33
Proximity effects and ortho interactions in 2,20-disubstituted diaryl amines have been reported104. Thus, phenazine, phenazine-N-oxide and carbazole were formed by loss of small neutral fragments, such as NO, NO2 and NO3ž , from the molecular ions, as illustrated by the formation of carbazole from 2,20 -dinitrodiphenylamine; see Scheme 34. Of particular interest is the loss of NO3ž , as demonstrated by high-resolution MS data and metastable ion spectra104.
|
−NO3 |
−NO |
+ |
|
|
+ |
|
||
NO2 |
O2 N |
|
|
|
|
|
−2 NO2 |
|
|
N |
−NO2 |
−NO2 |
N |
|
H |
||||
H |
|
|
||
|
|
|
SCHEME 34
The electron impact induced ortho effects in 2-nitro-substituted aromatic sulphides containing e.g. 2-pyridyl, 2-(5-methylthio-1,3,4-thiadiazolyl) moieties have been studied105, the 2,6-bis(nitrophenylthio)pyridines being typical representatives for the compounds studied105.
S |
N |
S |
NO2 |
|
NO2 |
NO2 |
|
NO2 |
S N S
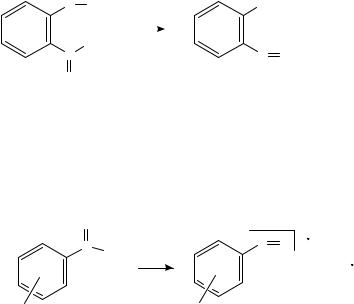
6. Mass spectrometry of nitro and nitroso compounds |
287 |
The decomposition of these compounds is strongly affected by several competing ortho- effects, due to the interaction of the nitro group(s) with neighbouring electron-deficient N-heterocyles. These ortho-effects can be grouped into three classes: (I) primary cyclization reactions by loss of small radicals, e.g. NO2 and OHž , (II) intra-molecular oxygen transfers from the nitro group to the heteocyclic ring systems and (III) primary skeletal rearrangements by intramolecular oxygen transfers followed by the extrusion of, e.g., SO and SO2105.
I. Protonated Nitro Compounds
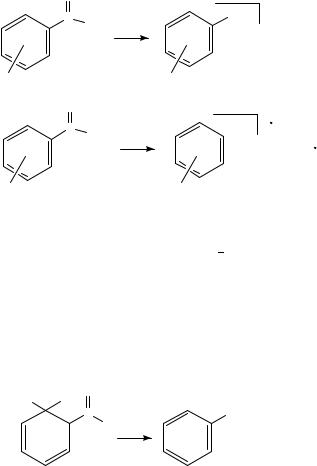
The chemical ionization MS of a selection of substitued nitrobenzenes have been studied106. The H2 chemical ionization MS appeared significantly more useful for characterization of, e.g., isomeric compounds than the corresponding methane spectra, apparently due to the higher internal energy deposited in the protonated molecule by the reaction with H3C , and consequently in the more extensive fragmentation106.
The nitroarenes with an ortho substituent bearing a hydrogen show loss of H2O from the protonated molecule. This fragment frequently turns out as the predominant ion in the MS. It should be noted that this fragmentation is absent in the corresponding meta and para isomers106. The H2O elimination resembles the characteristic loss of OHž from the molecular ions in the electron impact mass spectra (cf Section IV.D.1) and is as such indicative of protonation of a nitro group followed by [1,4] elimination of H2O106; see Scheme 35.
X H |
|
X+ |
+ |
|
+ H2 O |
|
||
OH |
|
N O |
N |
|
|
O |
|
|
SCHEME 35
The loss of OHž from the protonated molecule appears to be a general feature of nitroarenes; see Scheme 36. However, this process is particularly dominant for compounds with electron-donating substituents ortho or para to the nitro function. Thus, this fragmentation reaction can be rationalized in terms of protonation of the nitro group, the [MH OH]C fragment being stabilized by electron donation through the mesomeric effect of the substituent106.
O |
|
|
N |
+ |
N O + |
OH |
|
|
|
|
|
|
|
+ OH |
X |
|
X |
|
|
SCHEME 36 |
The loss of NO, HNO, NO2 and HNO2 moieties is all a result of fragmentations common for nitroarenes. The importance of these fragmentation paths appears to be more
288 |
Helge Egsgaard and Lars Carlsen |
pronounced for nitroarenes containing electron-donating substituents in the meta position than for those being substituted in the ortho or para positions. It should be noted that the observed loss of, e.g., NO2 may still occur from the nitro-protonated species. This is supported by the collision-induced fragmentations at 10 and 50 eV, where the loss of NO2 is replaced by loss of HNO2 as the internal energy of the fragmenting ion increases, in complete accordance with the expected increase of simple bond fissions at the expense of rearrangement reactions with increasing internal energy106. Thus, the MS/MS results are consistent with protonation predominantly of the nitro group. The thermodynamically favoured site of protonation of nitrobenzene was previously demonstrated to be the nitro function rather than the aromatic ring107; see Scheme 37.
O
N + OH + 
OH
+ NO
X X
O
N + + OH
+ NO2
X X
SCHEME 37
The sites of protonation of aromatic compounds, including the possible three mono fluoronitrobenzenes, have been studied by neutralization reionization mass spectrometry (NRMS)22. The NRMS experiments on the MDC species generated by D2 chemical ionization clearly indicated that the DC attachment takes place to the nitro group rather than to the aromatic ring, as evidenced by the abundant losses of ODž and DNO2 NO C ODž 22.
In addition, a number of unusual fragmentation reactions, specific for certain substituents, have been observed for the nitroarenes. Thus, the protonated 2-nitrotoluene apparently eliminates nitrosomethane108. This reaction is not observed for either the meta or the para isomers and, hence, clearly involves the interaction of the two adjacent groups; see Scheme 38.
|
O |
|
H |
CH3 |
O+ |
|
N |
|
|
+ |
O |
|
+ CH3 NO |
|
|
|
|
|
|
SCHEME 38 |
The dominant formation of m/z 78 in the fragmentation of the metastable ion of 2-cyanonitrobenzene has been rationalized by analogy to the HCN elimination from
