
Chen The electron capture detector
.pdfCOMPLEMENTARY THEORETICAL CALCULATIONS |
127 |
F were positive without correlation and relativistic effects. However, a simple statement of the variational principle was used to suggest that
The energy of the wave function for N electrons, over the normal nonrelativistic Hamiltonian describing their motion in the field of a finite number of nuclei fixed within a bounded region, is an upper bound to the ground Hartree Fock energy of an N þ 1 electron system in the identical nuclear field. . . . A generalization of this theorem holds, implying in particular that the electron affinities of one-center systems are always non-negative. [81]
Thus, the electron affinities of the rare gases, nitrogen, and group II elements are positive but small, and the AEa of the remaining elements should be larger than these.
The electron affinity of the hydrogen atom was calculated to chemical accuracy in 1930 using the variational method. A value of 0.74(4) eV is compared with the EvV of 0.75419(2) eV. This was obtained by applying the variational principle to approximate wave functions for the neutral and anion. In 1962 C. L. Pekeris used 444 parameters and obtained a value of 0.75421 eV. Until 1991 this was the most accurate and precise value for the electron affinity of the H atom [82–85]. The calculation of electron affinities of atoms beyond hydrogen were challenges to theoretical chemists until recently. The earliest calculations gave negative electron affinities for the first row elements, except for F and C. The problem was that the Hartree Fock method only considered the correlation of electrons with parallel spins [85].
As more experimental values of atomic electron affinities were obtained, the theoretical calculations agreed with experiment values for many of the elements. For example, the addition of electron correlation to the self-consistent field calculations were used to verify the electron affinities of Li, B, C, N, O, F, Na, Al, Si, S, Cl, K, and some of the transition metals in the 1960s. The standard deviation of these values from the EvV is about 0.15 eV, setting the negative values to zero. However, some of the predictions are higher than the current best experimental values by as much as 0.4 eV. By the 1980s the calculations were extended to higher atomic weights with about the same standard deviation. The maximum deviations were as large as 0.6 eV for some of the higher transition metals [85]. More recently, ab initio calculations of the Ea of the main group elements up to Cl have been improved so that the standard deviation of the values from experiment has been reduced to 0.03 eV [8].
The electron affinities of the atoms can also be estimated by semi-empirical procedures. For the main group elements the values for a given family should be relatively constant since they have the same outer electronic configuration. Except for nitrogen and oxygen, this is true. The values of the group IIA and IIB elements and the rare gases are expected to be close to zero. The experimental values for the halogens range from 3.1 eV to 3.6 eV and average 3.4 0.15 eV. Likewise, the values for H and the alkali metals range from 0.75 eV to 0.5 eV and average 0.55 0.05 eV. The Group IB elements—Cu, Ag, and Au—are another exception
128 COMPLEMENTARY EXPERIMENTAL AND THEORETICAL PROCEDURES
to this constant value. The values for Cu and Ag are the same, but that for Au is 1 eV higher. This is larger than the change from N to P, 0.75 eV, or O to S, 0.5 eV. The change from Pd to Pt represents the largest change from one row to another at 1.5 eV. The horizontal analysis can also be used to estimate electron affinities. Except for the first row the periodic behavior of the main group elements is about the same. In the first row the values for N and O are low, but fall in the expected order. The partial filling of the p shell at N is clearly responsible for the near-zero electron affinity.
6.7.2Polyatomic Molecules
The extension of the quantum mechanical calculations to polyatomic species introduces a further complication, the change in the geometry of the anion relative to that of the neutral. The valence-state electron affinities of the homonuclear diatomic molecules can be calculated from simple molecular orbital theory. For the H2 molecule there are two bonding electrons. In the molecular anion the additional electron goes into an antibonding orbital so that there is only one net bonding electron. Thus, the bond energy of the anion should be about one-half that of H2. Since the electron affinity of the H atom is less than one-half the bond energy of H2, the valence-state electron affinity will be negative. The equation is EaðH2Þ EaðHÞ ¼ DðH2ð ÞÞ DðH2Þ ¼ 12 DðH2Þ, which gives EAðH2Þ ¼ 2:75 0:75 eV or 2 eV. However, just as in the case of atoms, the most stable form of the anion will be an electron at some distance r and a hydrogen molecule. Quantum mechanical calculations give a molecule and ‘‘free’’ electron. This will result in a positive but small AEa. A similar situation occurs for the N2 molecule. In the case of N2 the number of bonding electrons is six, indicating a triple bond. The addition of another electron to an antibonding orbital reduces this to five. Thus, the bond dissociation energy of the anion is 5/6D(N2). When this is combined with the negligible electron affinity of the nitrogen atom, the valence-state electron affinity of N2 is 1/6D(N2) or 2 eV.
The valence-state electron affinities of the remaining homonuclear diatomic molecules can be estimated from the number of bonding and antibonding electrons and the bond dissociation energy of the neutral and electron affinity of the atom. The group I and group VII elements have a relative bond order of 12 (two net bonding electrons in the neutral and one in the anion); DeðX2 Þ=DeðX2Þ ¼ the net number of bonding electron in the ion divided by that of the neutral; and EaðX2; M2Þ ¼ 1=2DðX2; M2Þ EaðX; MÞ. Within this approximation the electron affinities of all the homonuclear diatomic molecules in a given family will have the same relative bond order. However, the experimental values for the group I and VII elements range from 0.55 to 1.0. The predicted value for groups III and IV are 1.5 and 1.25 but most experimental values are larger. This is primarily due to the additional correlation energy in the anion.
The simple molecular orbital theory of bonding in homonuclear diatomic molecules can be used to estimate the electron affinities of clusters. In these cases, there can be different geometries. The Cn clusters have been studied most extensively. In the case of the triatomic molecules, there are now two distances and one angle that
COMPLEMENTARY THEORETICAL CALCULATIONS |
129 |
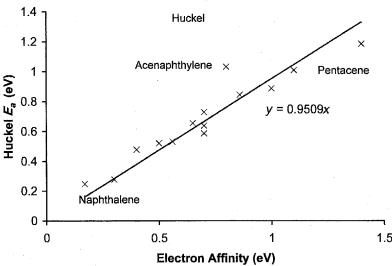
Figure 6.18 Precision and accuracy plot for Huckel Ea for aromatic hydrocarbons. The slope of the zero intercept line is less than 1, indicating systematic uncertainties [79].
can be different in the anion than in the neutral. The electron affinities of the molecules that are linear in the neutral and become bent in the anion illustrate these effects dramatically. The triatomic molecules CO2, CS2, COS, and N2O have been studied in the ECD. Many of these electron affinities are still in question. There are clearly two bound electronic states of the anion, the linear form and the bent form. The Ea can also be determined using CURES-EC. Many of the electron affinities of these molecules have also been calculated using density functional methods. Other small polyatomic molecules studied using the ECD and CURESEC are nitromethane, biacetyl, and SF6.
The calculations of the Ea for species containing fewer than six heavy atoms agree with experimental values to within less than 0.1 eV. However, for all but these smallest molecules the more rigorous techniques still give Ea that agree with experiment to better than 0.1–0.2 eV. The use of simple density functional theory for small radicals where the anion is a closed shell species and the neutral is an open shell gives absolute and standard deviations of about 0.12 eV. Where the neutral is a closed shell species and the anion an open shell species, the density functional theory give an average deviation of 0.2 to 0.3 eV. The standard deviations are greater than 0.15 eV [8]. Consequently, a need to find a simpler more accurate procedure for calculating the electron affinities of large organic molecules exists. The semi-empirical self-consistent field CURES-EC procedure is such a possibility.
Huckel theory was used to confirm the electron affinities of aromatic hydrocarbons determined with the ECD. Figure 6.18 shows the Ea for several aromatic hydrocarbons calculated from a linear correlation of the Huckel coefficients of the lowest unoccupied molecular orbital (LUMO) versus the EvV from gas phase
130 COMPLEMENTARY EXPERIMENTAL AND THEORETICAL PROCEDURES
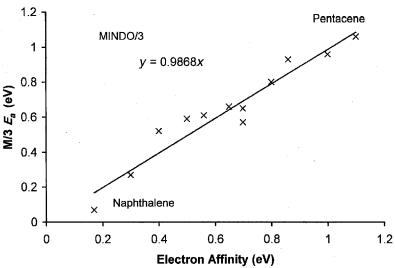
Figure 6.19 Precision and accuracy plot for MINDO/3 Ea for aromatic hydrocarbons. The slope of the zero intercept line is approximately 1, but there are outliers, [89].
measurements. The slope with zero intercept is 0.95. The greatest deviations are0.2 eV for pentacene and acenaphthylene. The standard deviation is 0.12 eV. The MINDO/3 self-consistent field procedures for calculating electron affinities represented a large advance in theoretical calculations. In Figure 6.19 the MINDO/3 semi-empirical self-consistent field calculated values for the above hydrocarbons are plotted against the EvV [39, 85–87]. The slope with a zero intercept becomes 1.00 and the standard deviation is reduced to 0.1 eV. These results are the basis for the use of CURES-EC to improve the calculated values and experimental electron affinities from half-wave reduction potentials [39]. Modern ab initio and density functional techniques have been applied to the calculation of the Ea of aromatic hydrocarbons, but insufficient values to test the methods exist [8]. The MNDO semi-empirical self-consistent field procedures have been used to calculate electron affinities. In the first calculations using the MNDO procedure it was concluded that the MNDO procedure reproduced the available experimental electron affinities of a variety of ‘‘delocalized’’ radicals with a mean error of0.4 eV [87]. There were some notable successes, such as the calculation of the electron affinity of benzoquinone, 1.62, 1.88 eV versus 1.860 eV, and TCNE, 3.06 eV versus 2.95 eV [85, 87]. Extensive calculations of electron affinities using the MNDO, AM1, and PM3 procedures did not occur until the development of CURES-EC.
The electron affinities of a series of substituted quinones have been calculated using the hybrid Hartree Fock/density functional B3LYP method with a 6-311G(3d,p) basis set. The precision and accuracy plot for the Ea obtained from

COMPLEMENTARY THEORETICAL CALCULATIONS |
131 |
Figure 6.20 Precision and accuracy plot for density functional Ea for the quinones in Figure 6.17. The slope of the zero intercept line is approximately 1 and the deviations are random [9].
these calculations is shown in Figure 6.20. However, these calculations have not been extended to enough molecules to test their predictive ability [9]. Calculations for 20 substituted nitrobenzenes have been carried out using the Hartree Fock procedure and the 6-31þG* basis set to find the optimized geometry for the reactants and products in the thermal charge transfer reactions: ABð Þ þ CD , CDð Þ þ AB, where AB is nitrobenzene. A good correlation between the calculated and experimental energies was obtained [88]. This represents an improvement over the ab initio calculations for hydrocarbons, but the values of the LUMO are still positive, implying a negative Ea. By displacing the LUMO and scaling to the Ea for nitrobenzene of 1.00 eV, the unit slope intercept is 0.0 eV and the standard deviation 0.13 eV. These are shown in the precision and accuracy plot of Figure 6.21. These values will be discussed further in Chapter 10. The success of this procedure suggests that the LUMO obtained from the semi-empirical SCF calculations could also be correlated to the experimental values. It is important to have theoretical methods that can be used to verify the experimental values because there are so few confirmed experimental Ea. The modified density functional calculations give good results for a limited number of quinones. The ab initio values for aromatic nitro compounds can be scaled to render accurate and precise predictions. However, these still require more computational time than simple SCF semiempirical calculations. This is especially true for the larger molecules important to organic, biological, and environmental chemistry.

132 COMPLEMENTARY EXPERIMENTAL AND THEORETICAL PROCEDURES
Figure 6.21 Precision and accuracy plot for scaled ab initio LUMO Ea for nitrobenzenes, benzonitriles, benzophenones, and benzaldehydes. The slope of the zero intercept line is approximately 1 and the deviations are random [91].
6.8 RATE CONSTANTS FOR ATTACHMENT, DETACHMENT, AND RECOMBINATION
The specific rate constants of interest to the ECD and NIMS are dissociative and nondissociative electron attachment, electron detachment, unimolecular anion dissociation, and electron and ion recombination. The reactions that have been studied most frequently are electron attachment and electron and ion recombination. To measure recombination coefficients, the electron concentration is measured as a function of time. The values are dependent on the nature of the positive and negative ions and most important on the total pressure in the system. Thus far few experiments have been carried out under the conditions of the NIMS and ECD. However, the values obtained under other conditions suggest that there is a limit to the bimolecular rate constant, just as there is a limit to the value of the rate constant for electron attachment. The bimolecular rate constants for recombination are generally large, on the order of 10 7 to 10 6 cc/molecule-s or 1014 to 1015 l/mole-s at about 1 atm pressure. Since the pseudo-first-order rate constants are approximately 100 to 1,000 s 1, the positive-ion concentrations in the ECD and NIMS are about 109 ions/cc.
The electron beam and electron swarm experiments [13] can also be used to determine attachment rate constants. However, these are determined as a function of energy and can then be extrapolated to thermal energy. Other techniques used to
RATE CONSTANTS FOR ATTACHMENT, DETACHMENT, AND RECOMBINATION |
133 |
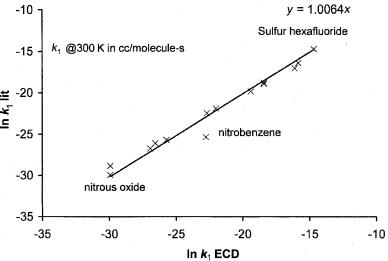
Figure 6.22 Precision and accuracy plot for rate constants determined by other techniques versus those determined by the ECD at room temperature [13, 91].
obtain rate constants for thermal electron attachment include the microwave stationary and flowing afterglow, microwave conductivity method, ion cyclotron method, electron cyclotron resonance method, electron density sampling method, and flowing afterglow Langmuir probe (FALP) method. In general, the rate constants are obtained by measuring the electron concentration as a function of time. A large body of data has been obtained using these techniques. The data are generally only reported at a single temperature [12–14, 89–107]. However, some have been investigated as a function of both electron temperature and the bulk temperature. These studies have been summarized by D. Smith and P. I. Spanel [106].
Figure 6.22 is a log plot of the ECD values versus those obtained by other techniques. If the value of the activation energy has been determined and a value of the rate constant has been measured by a different technique, then a value can be calculated for A1. Alternatively, a value of A1 equal to the DeBA could be assumed to calculate activation energies. When this is done, the range of activation energies is 0 kcal/mole to 15 kcal/mole, or 0 eV to 0.65 eV. In Chapter 11 this procedure will be applied to compounds of environmental interest. The activation energies measured in recent studies are equal to those obtained by the ECD [107]. The electron energy has been varied independently from the gross temperature. Significant differences for the types of energy variation were observed, in keeping with the earlier swarm studies of electron attachment cross-sections as a function of average energy [14, 106].
134 COMPLEMENTARY EXPERIMENTAL AND THEORETICAL PROCEDURES
6.9SUMMARY
The experimental methods for determining electron affinities and rate constants for thermal electron attachment that complement the ECD and NIMS methods of study have been summarized. The nominal uncertainties for each method of measuring electron affinities were established by comparing the values with the current evaluated values. By calibrating to the Ea of benzoquinone and maleic anhydride, the
Ea from the |
general TCT methods |
are |
(0.8 eV, benzophenone) < ( |
0.9 eV, |
C6F6) < (1.0 |
eV, nitrobenzene) < (1.1 |
eV, |
p-F-nitrobenzene) < (1.2 eV, |
phthalic |
anhydride) < (1.3 eV, m-Cl-nitrobenzene) < (1.4 eV, maleic anhydride) < (1.82 eV, naphthoquinone) < (1.85 eV, benzoquinone), all 0.1 eV. Some values are lower than the evaluated values and have been assigned to excited states. These are SF6, CS2, and NO from AMB, the ICR value for fluoroanil (2.45(5) eV), and the HPMS values for C6F6 (0.52(10) eV), CS2 (0.62(10) eV), azulene (0.70(10) eV), and anthracene (0.60(10) eV). The magnetron values for SF6, (1.4(2)) and (C6F6) (1.2(2)), and the AMB value for fluoranil (2.9(2) eV) are high but included in the weighted average values. The EnCT value of 1.8(3) eV for C6F6 is significantly larger than the EvV and included in the average.
The theoretical methods for calculating the electron affinities of atoms, diatomic molecules, and polyatomic molecules have been summarized and compared with the CURES-EC method for molecules. The density functional calculations of the electron affinities of substituted benzoquinones and scaled ab initio LUMO agree with the evaluated values for nitrobenzenes.
The rate constants for thermal electron attachment by alternative techniques are compared to those obtained with the ECD. A method of calculating activation energies for rate constants measured at a single temperature is suggested.
REFERENCES
1.Chen, E. C. M. and Wentworth, W. E. Mol. Cryst. Liq. Cryst. 1989, 171, 271.
2.Page, F. M. and Goode, G. C. Negative Ions and the Magnetron. New York: WileyInterscience, 1969.
3.Kebarle, P. and Chowhury, S. Chem. Rev. 1987, 87, 513.
4.Kleyn, A. W. and Moutinho, A. M. C. J. Phys. B 2001, 34, R1.
5.Dillard, J. G. Chem. Rev. 1973, 73, 700.
6.Jordan, K. D. and Burrow, P. D. Chem. Rev. 1987, 87, 557.
7.Reinstra-Kiracofe, J. C.; Tschumper, G. S.; Schaefer, H. F.; Nandi, S.; and Ellison, G. B.
Chem. Rev. 2002, 102, 231.
8.Reinstra-Kiracofe, J. C.; Barden, C. J.; Brown, S. T.; and Schaefer, H. F. J. Phys. Chem. A 2001, 105, 524.
9.Boesch, S. E.; Grafton, A. K.; and Wheeler, R. A. J. Phys. Chem. 1996, 100, 10083.
REFERENCES 135
10.Christodoulides, A. A.; McCorkle, D. L.; and Christophorou, L. G. ‘‘Electron Affinities of Atoms, Molecules and Radicals’’ in Electron-Molecule Interactions and Their Applications. New York: Academic Press, 1984.
11.National Institute of Standards and Technology (NIST). Chemistry WebBook, 2003. Available at http://webbook.nist.gov.
12.Caledonia, G. E. Chem. Rev. 1975, 75, 333.
13.Christodoulides, A. A.; McCorkle, D. L.; and Christophorou, L. G. ‘‘Electron Attachment Processes’’ in Electron-Molecule Interactions and Their Applications. New York: Academic Press, 1984.
14.Spanel, P.; Matejcik, S.; and Smith, D. J. Phys. B 1995, 28, 2941.
15.Stemmler, E. A. and Hites, R. A. Electron Capture Negative Ion Mass Spectra. New York: VCH, 1988.
16.Pack J. L. and Phelps, V. Phys. Rev. Lett. 1961, 6, 111.
17.Freeman, R. R. Ph. D. dissertation, University of Houston, 1971.
18.Van de Wiel, H. J. and Tommasen, H. J. Chromatogr. 1972, 71, 1.
19.Rolla, L. and Picardi, G. Atti. Accad. Linef. 1935, 2VI, 29, 128, 73.
20.Smith, H. and Sugden, T. M. Proc. Roy. Soc.(Lond.) 1952, A211, 31, 58.
21.Sutton, P. and Mayer, J. E. J. Chem. Phys. 1935, 3, 20.
22.Glockler, G. and Calvin, M. J. Chem. Phys. 1936, 4, 492.
23.Christophorou, L. G. and Datkos, P. G. Int. J. Mass Spectrom. Ion Proc. 1995, 149/150, 59.
24.Mock, R. S. and Grimsrud, E. P. J. Amer. Chem. Soc. 1989, 111, 2861.
25.Brinkman, E. A.; Gunther, E.; and Brauman, J. I. J. Chem. Phys. 1991, 95, 6185.
26.Smith, S. J. and Branscomb, L. M. J. Res. Nat. Bur. Stds. 1955, 55, 165.
27.Burch, D. S.; Smith, S. J.; and Branscomb, L. M. Phys. Rev. 1958, 112, 171.
28.Branscomb, L. M. (ed.). Photodetachment in Atomic and Molecular Processes. New York: Academic Press, 1962, p. 130.
29.Berry, R. S. and Reimann, C. W. J. Chem. Phys. 1963, 38, 1540.
30.Scheidt, J. and Weinkauf, R. Chem. Phys. Lett. 1997, 266, 201.
31.Chen, E. S. D. and Chen, E. C. M. J. Chromatogr. A 2002, 952, 173.
32.Oakes, J. M. and Ellison, G. B. Tetrahedron, 1986, 42, 6263.
33.Schiedt, J. and Weinkauf, R. Z. Natforsch. 1995, 50a, 1041.
34.Chen, E. C. M.; George, R.; Carr, S.; Wentworth, W. E.; and Chen, E. S. D. J. Chromatogr. A 1998, 811, 250.
35.Kraus, K.; Muller-Duysing, W.; and Neuert. Z. Natforsch. 1961, 16a, 1385.
36.Hughes, B. M.; Lifschitz, C.; and Tiernen, T. O. J. Chem. Phys. 1973, 59, 3162.
37.Compton, R. N.; Reinhardt, P. W.; and Cooper, C. D. J. Chem. Phys. 1975, 63, 3821.
38.Duncan, M. A. M.; Knight, M.; Negishi, Y.; Nagao, S.; Nakamura, Y.; Kato, A.; Nakajima, A.; and Kaya, K. Chem. Phys. Lett. 1999, 309, 49.
39.Chen, E. S. D.; Chen, E. C. M.; Sane, N.; Talley, L.; Kozanecki, N.; and Shultze, S. J. Chem. Phys. 1999, 110, 9319.
40.Wojnarvits, L. and Foldiak, G. J. Chromatogr. 1981, 206, 511.
136COMPLEMENTARY EXPERIMENTAL AND THEORETICAL PROCEDURES
41.Crocker, L.; Wang, T. B.; and Kebarle, P. J. Amer. Chem. Soc. 1993, 115, 7818.
42.Chen, E. C. M.; Carr, S.; Wentworth, W. E.; and Chen, E. S. D. J. Chromatogr. A 1998, 827, 91.
43.Desfrancois, C.; Periquet, V.; Lyapustina, S. A.; Lippa, T. P.; Robinson, D. W.; Bowen,
K.H.; Nonaka, H.; and Compton. J. Chem. Phys. 1999, 111, 4569.
44.Chen, E. C. M.; Welk, N.; Chen, E. S. D.; and Wentworth, W. E. J. Phys. Chem. A 1999, 103, 9072.
45.Compton, R. N.; Carman Jr.; Desfrancois, C.; Abdoul-Carmine, H.; Schermann, J. P.; and Hendricks, J. H. J. Chem. Phys. 1996, 105, 3472.
46.Lecomte, F.; Carles, S.; Desfrancois, C.; and Johnson, M. A. J. Chem. Phys. 2000, 113, 10973.
47.Lyons, L. E. and Palmer, L. D. Aust. J. Chem. 1976, 29, 1919.
48.Lyons, L. E. and Palmer, L. D. Int. J. Mass Spectrom. Ion Phys. 1975, 16, 431.
49.Lyons, L. E. and Palmer, L. D. Chem. Phys. Lett. 1973, 21, 442.
50.Wenthold, P. G.; Hrovat, D. A.; Borden, W. T.; and Lineberger, W. C. Science 1996, 272, 1456.
51.Scheidt, J.; Weinkauf, R.; Neumark, D. M.; and Schlag, E. W. Chem. Phys. 1998, 123, 511.
52.Bartmess, J. E. and McIver, R. T. ‘‘The Gas Phase Acidity Scale,’’ in Gas Phase Ion Chemistry, Vol. 2, edited by M. T. Bowers. New York: Academic Press, 1979, p. 88.
53.Henglein, A. and Muccini, G. A. J. Chem. Phys. 1959, 31, 1426.
54.Kraus, K.; Muller-Duysing, W.; and Neuert, H. Z. Naturfor. 1961, 16A, 1385.
55.Goldan, P. D.; Schmeltekopf, A. L.; Schiff, H. I.; and Ferguson, E. E. J. Chem. Phys. 1966, 44, 4095.
56.Rains, L. J.; Moore, H. W.; and McIver, R. T. J. Chem. Phys. 1978, 68, 3309.
57.Fukuda, E. K. and McIver, R. T. In Lecture Notes in Chemistry, edited by H. Hartman and
K.P. Wanzek. Berlin: Springer, 1982, p. 164.
58.Fukuda, E. K. and McIver, R. T. J. Chem. Phys. 1982, 77, 4942.
59.Fukuda, E. K. and McIver, R. T. J. Amer. Chem. Soc. 1985, 107, 2291.
60.Burinsky, D. J.; Fukuda, E. K.; and Campana, J. E. J. Amer. Chem. Soc. 1984, 106, 2270.
61.Chen, G.; Cooks, R. G.; Corpuz, E.; and Scott, L. T. J. Amer. Soc. Mass Spectrom. 1996, 7, 619.
62.Denault, J. W.; Chen, G. D.; and Cooks, R. G. J. Amer. Soc. Mass Spectrom. 1998, 9, 1141.
63.Chen, G. and Cooks, R. G. J. Mass Spectrom. 1997, 32, 333.
64.Chupka, W. A.; Berkowitz, J.; and Gutman, D. J. Chem. Phys. 1971, 55, 2724.
65.Hughes, B. M.; Lifschitz, C.; and Tiernan, T. O. J. Chem. Phys. 1973, 59, 3162.
66.Lifshitz, C.; Tiernan, T. O.; and Hughes, B. M. J. Chem. Phys. 1973, 59, 3182.
67.Laramee, J. A.; Mazurkiewicz, P.; Berkout, V.; and Deinzer, M. L. ‘‘Discrete Electron Capture Negative Ion Mass Spectrometry’’ in Encyclopedia of Analytical Chemistry. New York: Wiley, 2000.
68.Kline, L. E.; Davies, D. K.; Chen, C. L.; and Chantry, P. J. J. Appl. Phys. 1979, 50, 6789.
