
j.electacta.2013.03.143
.pdf
Accepted Manuscript
Title: Modeling of the Electrochemical Impedance
Spectroscopic Behavior of Passive Iron Using a Genetic
Algorithm Approach
Author: Samin Sharifi-Asl Matthew L. Taylor Zijie Lu
George R. Engelhardt Bruno Kursten Digby D. Macdonald
PII: |
S0013-4686(13)00566-5 |
DOI: |
http://dx.doi.org/doi:10.1016/j.electacta.2013.03.143 |
Reference: |
EA 20251 |
To appear in: |
Electrochimica Acta |
Received date: |
11-9-2012 |
Revised date: |
25-3-2013 |
Accepted date: |
27-3-2013 |
Please cite this article as: S. Sharifi-Asl, M.L. Taylor, Z. Lu, G.R. Engelhardt, B. Kursten, D.D. Macdonald, Modeling of the Electrochemical Impedance Spectroscopic Behavior of Passive Iron Using a Genetic Algorithm Approach, Electrochimica Acta (2013), http://dx.doi.org/10.1016/j.electacta.2013.03.143
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.

Modeling of the Electrochemical Impedance Spectroscopic Behavior of Passive Iron Using a Genetic Algorithm Approach
Samin Sharifi-Asl1, Matthew L. Taylor1, Zijie Lu2, George R. Engelhardt3, Bruno Kursten4, and 1 Digby D. MacdonaldManuscript5,6,*
Center for Electrochemical Science and Technology
Department of Materials Science and Engineering
Pennsylvania State University
University Park, PA 16802, USA.
2Ford Motor Company
Dearborn, MI, 48120
3OLI Systems
108 American Road
Morris Plains, NJ07950-2443, USA
4 SCKCEN
Boeretang 200 BE-2400 Mol BELGIUM
5 Department of Materials Science and Engineering
AcceptedUniversity of California at Berkeley
Berkeley, CA 94720
6Chair Professor
Center for Research Excellence in Corrosion Research Institute
King Fahd University of Petroleum and Minerals
Dhahran 31261, Saudi Arabia
Abstract
In order to predict the general corrosion damage to metals and alloys, development of deterministic models and the acquisition of values for various model parameters are of paramount importance. In the present work, the Point Defect Model (PDM) was further developed to account for the properties of the passive film on pure iron in deaerated solutions.
1
Corresponding author. E-mail: macdonald@berkeley.edu Telephone: (814) 360-3858
Page 1 of 57

The model parameter values were extracted from the electrochemical impedance spectroscopic (EIS) data collected for iron in borate buffer solution [0.3 M H3BO3 + 0.075 M
Na2B4O7, pH = 8.15 at 21 oC] + 0.001 M EDTA [Ethylenediaminetetraacetic acid, EDTA,
the formation of the outer layer of the passive film, therefore rendering the barrier layer amenable to direct examination. Comparison of the experimental and calculated data demonstrates that the impedance model based on the PDM provides a good account of the growth of the passive film on iron and the extracted model parameters can be used to predict the corrosion evolution of the sample as a function of time.
disodium salt] at 21 oC by optimization of the PDMManuscripton the experimental EIS data using an Genetically-inspired, Differential Evolution Algorithm (GDEA). EDTA effectively suppresses
Keyword: Point Defect Model, Genetic Algorithm, Complex optimization, Passivity, EIS
1. IntroductionAccepted
The growth of the passive film on iron in neutral and alkaline buffer solutions has been extensively investigated in the past [1-13]. A borate buffer solution with a pH value close to neutral or slightly alkaline (e.g., pH = 8.4) was usually chosen in these prior studies, partly because the current density in both the active and passive regions are much less than those observed in acidic solutions (pH < 7 at 25 oC), and hence lead to much less roughening of the electrode surface.
It is commonly accepted that the passive film on iron is an n-type semiconductor [5, 6] because the formation, ejection, and the transport of metal interstitials through the passive film are the principal electrochemical phenomena occurring in the passive state. Theory [12-17]
2
Page 2 of 57

shows that the passive current density should be independent of voltage, provided that no change in oxidation state occurs in the cations being transmitted through the barrier layer and being ejected at the barrier layer/solution (bl/s) interface or in those cations resulting from dissolution
of the film. |
Manuscript |
|
The formation of the passive film on iron has been explained by a variety of mechanisms, including the ion-migration mechanism [2] and the later, generalized high field model (HFM) [12]. More recently, the Point Defect Model (PDM) was developed by Macdonald and his coworkers to provide an atomic scale description of the interfacial processes that lead to passivity and passivity breakdown [13-17], and this model has been shown to be consistent with the steady-state properties of the passive state on iron. In contrast to the other mechanisms, the PDM predicts the existence of steady states in film thickness and current, and accounts for the
linear dependencies of the steady-state film thickness on potential and pH, all of which are
of values for the model parameters using a Genetically-inspired, Differential Evolution Algorithm (GDEA) [18] in conjunction with barrier layer thickness data obtained using the Spectroscopic Ellipsometric (SE) method [19] and with available data in literature.
observed experimentally.AcceptedThe objective of the current work was the development of an impedance model for the growth of the passive film on iron based upon the PDM and extraction
2. Point Defect Model
The Point Defect Model was developed by Macdonald and coworkers as a mechanistically-based model that could be tested analytically against experiment [20-22]. The PDM is now highly developed and to our knowledge, there are no known conflicts with experiment, where confluence between theory and experiment has been first demonstrated.
3
Page 3 of 57

Indeed, the model has predicted new phenomena that have subsequently been observed, including the photo-inhibition of passivity breakdown (PIPB) [13,23-25], and has provided a theoretical basis for designing new alloys from first principles [26,27]. The PDM has been
previously used to interpret electrochemical impedanceManuscriptdata by optimizing the model on the experimentally-determined real and imaginary components of the interphasial (metal/passive
film/solution) impedance, with considerable success [28-33]. An earlier version of the model has been extensively used to analyze data obtained in this program for carbon steel in simulated concrete pore water and these analyses will be discussed at length in a later paper. With the exception of one recent publication [34], all of the previous work from this laboratory used the commercial DataFit [35] software for optimization, which employed the Levenberg-Marquardt [36] method of minimization, in order to optimize the model onto the experimental data. The optimization work described below was performed using the same model as previous work; however, the Acceptedoptimization was performed with a newer method of optimization, Differential Evolution (DE), using custom software [37], which solves many of the issues associated with parameter optimization of functions of this type. The quality of solution is vastly improved (several orders of magnitude reduction in the chi-squared error over gradient-based methods). An overview of Evolutionary Algorithm methods is presented in Ref. [38]. Although gradientbased methods are computationally much faster than evolutionary methods, such as DE, without operator experience and the requirement for non-intuitive knowledge about a highly dimensional system, they are not operationally more efficient. The man-hours saved more than makes up for any shortcomings in terms of computational speed. Parenthetically, we note that the inclusion of reversible reactions will allow the PDM to also account for the reduction of the passive films, albeit at a considerable cost in mathematical complexity.
4
Page 4 of 57

Figure 1. (PDM Reactions)
The physico-chemical basis of the PDM is shown in Figure 1. Briefly, the model postulates that defect generation and annihilation reactions occur at the metal/barrier layer (m/bl) and the barrier layer/outer layer (bl/ol) interfaces and that these reactions essentially establish the point defect concentrations within the barrier layer. Analytical expressions for the rate constants
for the reactions, as derived by the method of partial charges, are summarized in Table 1.
Table 1. (Rate constants)
The electron current density, I, which is sensed in an external circuit, is given by:
I F k1CvL |
k2 |
k3 ( )k4 |
( )k5Ci0 ( |
)k7 |
(1) |
|
|
whereC L |
is |
the |
concentration of |
cation vacancies |
at the m/bl |
interface and C 0 |
is the |
|
|
|
|
|
|
i |
|
concentration of cation interstitials at the bl/ol interface.ManuscriptNote that Equation 1 does not depend |
|||||||
upon the concentrationAcceptedof oxygen vacancies or upon the rate constant for Reaction (6), Figure 1.
Thus, no relaxations in the impedance response involve oxygen vacancies, but this is essentially an artifact of considering Reaction (3), Figure 1, to be irreversible. If this reaction was considered to be reversible, then a relaxation involving oxygen vacancies would be present. Furthermore, the concentration of H+ is considered to be constant, corresponding to a wellbuffered solution, and is included in the definition of k7, as indicated in Equation 4.
Using the method of partial charges, the rate constants for the reactions are found to be of
the form: |
|
ki ki0 exp ai (V Rol I ) bi L , i = 1, 2, 3 |
(2) |
5
Page 5 of 57

ki ki0 exp ai (V Rol I ) , i = 4, 5 |
(3) |
and
specific resistance of the outer layer. We recognize,Manuscripthowever, that in the experiments reported in
|
|
|
0 |
exp a |
|
|
C |
H |
|
n |
|
k |
|
k |
(V R |
|
|
|
|
(4) |
|||
|
|
|
I ) |
|
|
|
|||||
|
7 |
|
7 |
7 |
|
ol |
C |
0 |
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
where n is the kinetic order of barrier layer dissolution with respect to H+. In deriving these
expressions theoretically, it is assumed that a resistive outer layer, Rol, exists on the surface of the
barrier layer and that the passive current flows through the barrier layer to a remote cathode,
which is the normal experimental configuration. Because of this, the potential that exists at the
bl/ol interface must be corrected from that applied at the reference electrode located at the outer
layer/solution interface by the potential drop across the outer layer, where Rol (Ω cm2) is the
this paper, EDTA was added to the solution to suppress the formation of the outer layer, so that
Rol = 0 and hence the impact of the outer layer is moot. It is included in the theory reported here only for the sake of completeness.
Let us assume that the applied potential changes sinusoidally around some mean value
(V ) in accordance with Equation (5):
V V V V Vej t |
|
|
(5) |
Accepted |
The bar over a letter refers to the |
||
where ω is an angular frequency and |
V |
is the amplitude. |
|
corresponding value under steady-state conditions. Accordingly, in the linear approximation
6
Page 6 of 57

have the following response f f fe j t , where f represents current density, I, and values on
which I depends, namely, L, C0 |
, |
C L , and the various rate constants. |
|||
|
|
|
i |
|
|
|
Our task, then, is to calculate the faradic admittance, YF, which is defined as: |
||||
Y 1 |
I |
I |
|
Manuscript |
|
|
(6) |
||||
F |
ZF |
V |
V |
|
|
where ZF is the faradic impedance. Note that I, is a function of the potential at the bl/ol interface (U), but the potential that is modulated is that at the outer layer/solution (ol/s) interface (V), or close to it, depending upon the exact placement of the tip of the Luggin probe. The two potentials are related by
U V Rol I |
|
|
|
|
|
(7) |
|
||||||
It is evident that, |
|
|
|
|
|
|
|||||||
1 |
|
|
1 |
|
1 |
or |
Y |
YF0 |
|
(8) |
|
||
|
|
|
|
|
|
|
|
||||||
Y |
|
|
Y 0 |
|
R |
|
|
F |
1 R Y 0 |
|
|
|
|
F |
|
|
|
|
|
|
|
|
|||||
|
|
|
F |
|
ol |
|
|
ol F |
|
|
|
||
where |
Y |
0 |
is |
the |
admittance calculated in the absence of the outer layer, assuming that the |
||||||||
|
|
|
|
F |
|
|
|
|
|
|
|
|
|
potential at the bl/ol interface is U under steady-state conditions. We see that Y |
Y 0 |
as Rol → |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
F |
F |
|
0 and Y |
1/ R |
|
for Y 0 |
; that is, the interphasial impedance becomes controlled by the |
|||||||||
|
|
|
|
F |
|
|
ol |
|
F |
|
|
|
|
|
|
|
|
|
|
|
Accepted |
|
|
|
|||
outer layer in the limit of an infinitely large outer layer specific resistance or an infinitely small barrier layer admittance.
7
Page 7 of 57
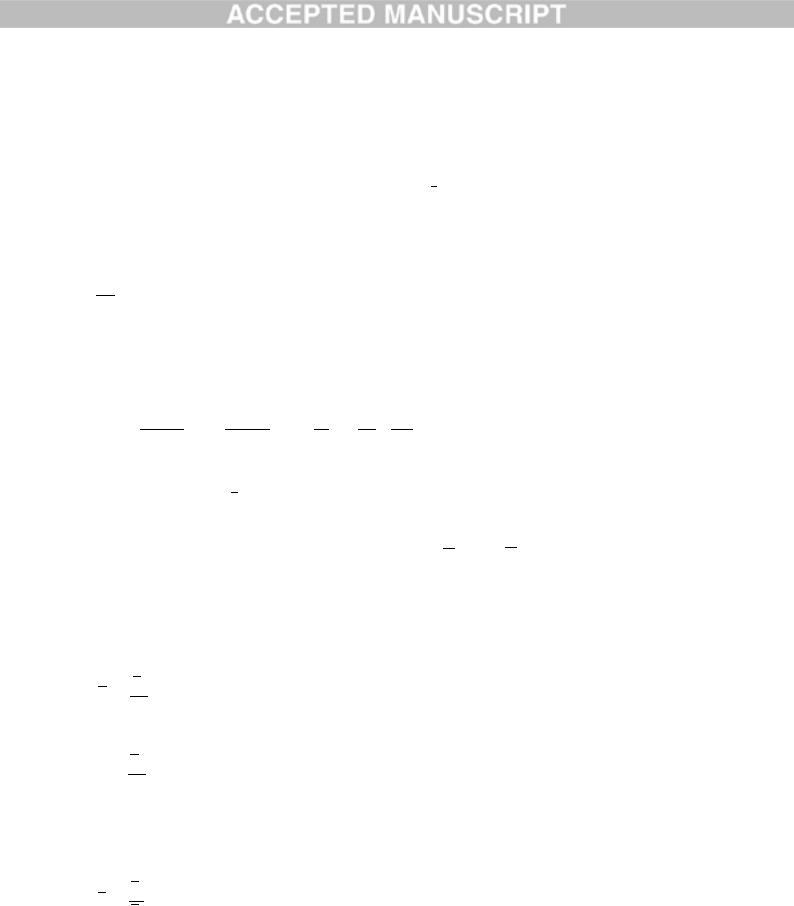
The values of U and other steady state values can be easily calculated. Assuming some
arbitrary value of U , we can immediately calculate ki , i = 4, 5, 7 from Equations (3) and (4).
From the rate equation for the change in thickness of the barrier layer, which is written as
dL |
k3 k7 |
|
|
|
|
|
|
|
|
|
|
Manuscript |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
(9) |
|||||||
dt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
we have k3 |
k7 , i.e. |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
a |
|
a |
|
|
C |
|
|
C |
|
1 |
|
|
0 |
C |
|
n |
|
|
|
L |
|
7 |
b |
|
3 |
U |
7 |
|
3 pH |
b |
ln |
7 |
|
H |
|
|
(10) |
|||
SS |
|
|
|
|
|
|
|
b |
|
|
k |
0 |
C |
0 |
|
|
|
|||
|
|
|
3 |
|
|
|
|
|
|
3 |
|
3 |
|
|
3 |
H |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
After that, the values ki |
|
(i = 1, 2) can be calculated by using Equations (2). |
||||||||||||||||||
|
The |
values |
of |
|
the |
steady-state |
concentrations C 0 |
and C L |
(concentrations of metal |
|||||||||||
|
|
|
|
|
Accepted |
|
i |
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
||||||||||||
interstitials at the bl/ol interface and oxygen vacancies at bl/ol interface) can be found by |
||||||||||||||||||||
equating the rates at each location to yield: |
|
|
|
|
|
|
||||||||||||||
CL |
k4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(11) |
v |
k1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C0 |
k2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(12) |
i |
k5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
and |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C0 |
k3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(13) |
O |
k6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8
Page 8 of 57

respectively. Equations (11) to (13) follows from the conditions that steady state fluxes of cation vacancies, cation interstitials, and oxygen vacancies, respectively, are constants (do not depend upon position inside the barrier layer).
i.e. we calculate the dependence I (V ) . As the actualManuscriptvalue of U , we will choose the value at which V equals the prescribed value, because no outer layer is assumed to exist in the
Finally, we calculate the values of
I F k1CvL |
k2 |
k3 ( )k4 |
( )k5Ci |
0 |
( )k7 |
(14) |
and |
|
|
|
|
|
|
V U Rol I |
|
|
|
|
|
(15) |
experiment. Practically, the task is reduced to the solution of the single equation V U Rol I (AcceptedU ) relative to the unknown value U (the voltage at the bl/ol interface).
We see that, if we have a code for calculating the admittance of the system in the absence of the outer layer, YF0 , we can calculate the admittance in the presence of the outer layer, YF , by using Equation (8), assuming that YF0 is calculated at the steady state applied potential that equals U (but not V ).
2.1. Calculation of YF0 .
As follows from Equation (1) we have in the linear form:
Y 0 |
|
I |
|
I |
I |
|
I |
L |
I L |
C L |
I 0 |
C 0 |
(16) |
U |
U |
|
L U |
v |
i |
||||||||
F |
|
|
|
U |
|
v |
U |
i U |
|
||||
9
Page 9 of 57
