
A Dictionary of Science
.pdf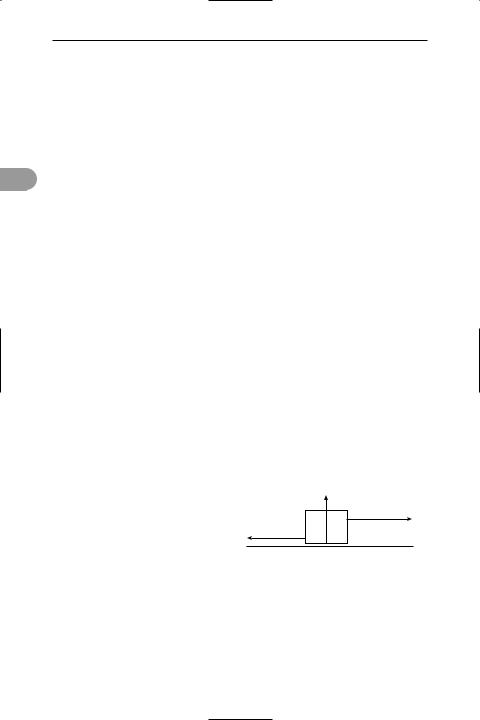
freezing mixture |
336 |
f
lows the visualization of the interior of plasma membranes and organelles. Cells are frozen at –196°C and cracked so that the plane of fracture runs through the middle of *lipid bilayers, separating the two halves. The exposed surfaces are then coated with carbon and platinum and the organic material is digested with enzymes (freeze etching), leaving a carbon–platinum replica of the fractured surface, which can be examined using the microscope.
freezing mixture A mixture of components that produces a low temperature. For example, a mixture of ice and sodium chloride gives a temperature of –20°C.
freezing-point depression See depres-
sion of freezing point.
Frenkel defect See crystal defect (Feature).
freon See chlorofluorocarbon.
frequency Symbol f or ν. The rate of repetition of a regular event. The number of cycles of a wave, or some other oscillation or vibration, per second is expressed in *hertz. The frequency ( f ) of a wave motion is given by f = c/λ, where c is the velocity of propagation and λ is the wavelength. The frequency associated with a quantum of electromagnetic energy is given by f = E/h, where E is the quantum’s energy and h is the Planck constant.
frequency modulation (FM) See modulation; radio.
fresnel A unit of frequency equal to 1012 hertz. In SI units this is equal to 1 terahertz (THz). It was named after the French physicist A. J. Fresnel (1788–1827).
Fresnel diffraction A form of *diffraction in which the light source or the receiving screen, or both, are at Ünite distances from the diffracting object, so that the wavefronts are not plane, as in *Fraunhofer diffraction. It was studied by A. J. Fresnel.
Fresnel lens A lens with one face cut into a series of steps. It enables a relatively light and robust lens of short focal length though poor optical quality to be used in projectors (as condenser lenses), searchlights, spotlights, car headlights,
and lighthouses. Such lenses have been made Üne enough to serve as magniÜers for reading small print.
friction The force that resists the motion of one surface relative to another with which it is in contact. For a body resting on a horizontal surface there is a normal contact force, R, between the body and surface, acting perpendicularly to the surface. If a horizontal force B is applied to the body with the intention of moving it to the right, there will be an equal horizontal friction force, F, to the left, resisting the motion (see illustration). If B is increased until the body just moves, the value of F will also increase until it reaches the limiting frictional force (FL), which is the maximum value of F. FL is then equal to µsR, where µs is the coefÜcient of static friction, the value of which depends on the nature of the surfaces. Once the body is moving with constant velocity, the value of F falls to a value Fk, which is equal to µkR, where µk is the coefÜcient of kinetic friction. Both µs and
µk are independent of the surface area of the body unless this is very small and µk is almost independent of the relative velocity of the body and surface.
Friction occurs because surfaces, however smooth they may look to the eye, have many microscopic humps and crests. Therefore the actual area of contact is very small, and the consequent very high pressure leads to local pressure welding of the surfaces. During motion the welds are broken and remade continually. See also
rolling friction.
R
B
F = µR
Friction
Friedel–Crafts reaction A type of reaction in which an alkyl group (from a haloalkane) or an acyl group (from an acyl halide) is substituted on a benzene ring (see illustration). The product is an alkylbenzene (for alkyl substitution) or an alkyl aryl ketone (for acyl substitution). The reactions occur at high temperature (about

337 |
froth flotation |
CH3
+CH3CI
benzene |
chloromethane |
methylbenzene |
|
|
(toluene) |
O
C CH3
+CH3COCI
benzene |
ethanoyl chloride |
phenyl methyl |
|
|
ketone |
f
Friedel–Crafts reaction. Examples of methylation (top) and acetylation (bottom).
100°C) with an aluminium chloride catalyst. The catalyst acts as an electron acceptor for a lone pair on the halide atom. This polarizes the haloalkane or acyl halide, producing a positive charge on the alkyl or acyl group. The mechanism is then electrophilic substitution. Alcohols and alkenes can also undergo Friedel– Crafts reactions. The reaction is named after the French chemist Charles Friedel (1832–99) and the US chemist James M. Crafts (1839–1917).
Frisch, Karl von See lorenz, konrad zacharias.
frogs See amphibia.
frond See megaphyll.
front (in meteorology) The sloping interface between two *air masses of different origins and thermal characteristics (e.g. temperature and humidity). Where the air masses come together the warm air, being lighter, rises and slopes over the colder air. Distinctive weather phenomena are associated with fronts. At a warm front (the boundary zone at the front of the warm sector of a *depression), warm air is overtaking cold air and rising above it with a slope of about 1:150. This is associated with cirrus clouds followed by cirrostratus, altostratus, then nimbostratus clouds, as the warm front passes. At a cold front, which is usually found to the rear of a de-
pression, warm air is forced to rise by an advancing undercutting wedge of cold air. This is accompanied by a sudden veering of the wind, a fall in temperature, and heavy rainfall. An occluded front (or occlusion) occurs when a cold front overtakes a warm front in an atmospheric depression.
frontal lobe The anterior part of each cerebral hemisphere, which is associated with the higher mental functions, such as abstract thought.
frontier-orbital theory A theory of the reactions of molecules that emphasizes the energies and symmetries of frontier orbitals: the two orbitals in a molecule that are the occupied orbital of highest energy and the unoccupied orbital of lowest energy. Frontier orbital theory was developed by the Japanese chemist Kenichi Fukui (1919–98) in the 1950s and is an alternative approach to the *Wood- ward–Hoffmann rules. It has been very successful in explaining such reactions as the *Diels–Alder reaction.
froth Ûotation A method of separating mixtures of solids, used industrially for separating ores from the unwanted gangue. The mixture is ground to a powder and water and a frothing agent added. Air is blown through the water. With a suitable frothing agent, the bubbles adhere only to particles of ore and carry
fructification |
338 |
them to the surface, leaving the gangue particles at the bottom.
fructiÜcation See sporophore.
fructose (fruit sugar; laevulose) A simple sugar, C6H12O6, stereoisomeric with glucose (see monosaccharide). (Although natural fructose is the d-form, it is in fact laevorotatory.) Fructose occurs in green plants, fruits, and honey and tastes sweeter than sucrose (cane sugar), of
fwhich it is a constituent. Derivatives of fructose are important in the energy metabolism of living organisms.
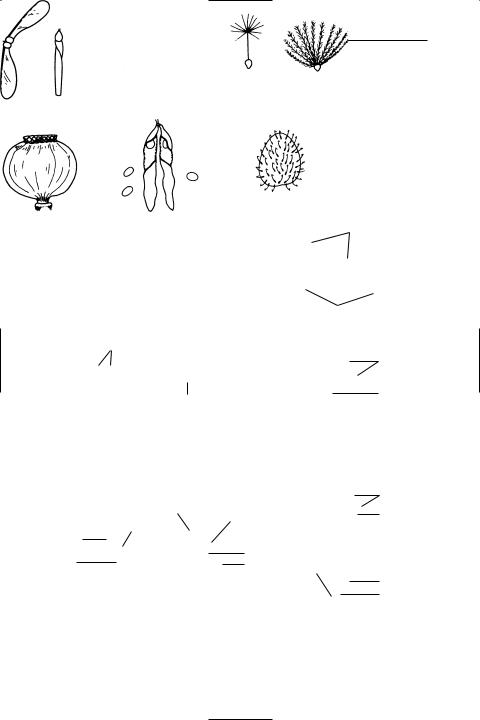
fruit The structure formed from the ovary of a Ûower, usually after the ovules have been fertilized. It consists of the fruit wall (see pericarp) enclosing the seed(s). Other parts of the Ûower, such as the receptacle, may develop and contribute to the structure, resulting in a false fruit (see pseudocarp). The fruit may retain the seeds and be dispersed whole (an indehiscent fruit), or it may open (dehisce) to release the seeds (a dehiscent fruit). Fruits are divided into two main groups depending on whether the ovary wall remains
sycamore
ash
samaras
pores through which seeds are dispersed
dispersed seeds
capsule (poppy) |
pod (laburnum) |
parachute of hairs
dandelion
thistle
fruit and seed
cypselas
hooked bracts
fruit
achenes in axils of hooked bracts (burdock)
Dry fruits: seeds dispersed by wind, water, or other mechanical means
|
|
achenes |
|
epicarp |
stony |
receptacle |
|
|
endocarp |
||
|
|
||
seed |
|
|
|
epicarp |
|
|
|
|
seed |
strawberry |
|
fleshy |
|
||
fleshy |
seed |
||
mesocarp |
mesocarp |
||
|
|
receptacle |
|
berry (grape) |
|
pericarp |
|
drupe (cherry) |
|||
(core) |
|||
apple (pome) pseudocarps (false fruits)
Succulent edible fruits: seeds dispersed by animals

339 |
fuller’s earth |
dry or becomes Ûeshy (succulent). Succulent fruits are generally dispersed by animals and dry fruits by wind, water, or by some mechanical means. See illustration.
See also composite fruit.
fruit Ûy See drosophila.
fruit sugar See fructose.
frustration A situation in which there are competing interactions in a system. For example, in a *spin glass the magnetic atoms are subject to both ferromagnetic and antiferromagnetic interactions. Frustration also occurs in the folding of large polymer molecules due to parts of the molecule getting in each other’s way. It is thought that proteins fold readily to speciÜc shapes because they have evolved to minimize this type of steric frustration.
frustum A solid Ügure produced when two parallel planes cut a larger solid or when one plane parallel to the base cuts it.
FSH See follicle-stimulating hormone.
FTP (Üle transfer protocol) The protocol used on the *Internet for transferring Üles (downloading or uploading) from one computer to another.
fucoxanthin The major *carotenoid pigment present, with chlorophyll, in the brown algae (see phaeophyta).
fuel A substance that is oxidized or otherwise changed in a furnace or heat engine to release useful heat or energy. For this purpose wood, vegetable oil, and animal products have largely been replaced by *fossil fuels since the 18th century.
The limited supply of fossil fuels has encouraged the development of *renewable energy sources.
fuel cell A cell in which the chemical energy of a fuel is converted directly into electrical energy. The simplest fuel cell is one in which hydrogen is oxidized to form water over porous sintered nickel electrodes. A supply of gaseous hydrogen is fed to a compartment containing the porous anode and a supply of oxygen is fed to a compartment containing the porous cathode; the electrodes are separated by a third compartment containing a hot alkaline electrolyte, such as potas-
sium hydroxide. The electrodes are porous to enable the gases to react with the electrolyte, with the nickel in the electrodes acting as a catalyst. At the anode the hydrogen reacts with the hydroxide ions in the electrolyte to form water, with the release of two electrons per hydrogen molecule:
H2 + 2OH– → 2H2O + 2e–
At the cathode, the oxygen reacts with
the water, taking up electrons, to form f hydroxide ions:
½O2 + H2O + 2e– → 2OH–
The electrons Ûow through an external circuit as an electric current. The device is a more efÜcient converter of electric energy than a heat engine, but it is bulky and requires a continuous supply of gaseous fuels. Their use to power electric vehicles is being actively explored. The second generation of fuel cells uses molten salts, particularly lithium or potassium carbonate, as electrolytes. The third generation uses solid conducting ionic oxides. See also biochemical fuel cell.
fuel element See nuclear reactor.
fugacity Symbol f. A thermodynamic function used in place of partial pressure in reactions involving real gases and mixtures. For a component of a mixture, d(lnf ) = dµ/RT, where µ is the chemical potential. It has the same units as pressure and the fugacity of a gas is equal to the pressure if the gas is ideal. The fugacity of a liquid or solid is the fugacity of the vapour with which it is in equilibrium. The ratio of the fugacity to the fugacity in some standard state is the *activity. For a gas, the standard state is chosen to be the state at which the fugacity is 1. The activity then equals the fugacity.
fullerene See buckminsterfullerene.
fullerite See buckminsterfullerene.
fuller’s earth A naturally occurring clay material (chieÛy montmorillonite) that decolorizes oil and grease. In the past raw wool was cleaned and whitened by kneading it in water with fuller’s earth; a process known as fulling. Fuller’s earth is now widely used to decolorize fats and oils and also as an insecticide carrier and
full-wave rectifier |
340 |
drilling mud. The largest deposits occur in the USA, UK, and Japan.
full-wave rectiÜer See rectifier.
fulminate See cyanic acid.
fulminic acid See cyanic acid.
fumaric acid See butenedioic acid.
function Any operation or procedure that relates one variable to one or more
fother variables. If y is a function of x, written y = f(x), a change in x produces a change in y, and if x is known, y can be determined. x is the independent variable and y is the dependent variable.
functional A function of a function. Functionals are used very extensively in the quantum-mechanical many-body
problem, statistical mechanics, and quantum Üeld theory.
functional group The group of atoms responsible for the characteristic reactions of a compound. The functional group is –OH for alcohols, –CHO for aldehydes, –COOH for carboxylic acids, etc.
fundamental See harmonic.
fundamental constants (universal constants) Those parameters that do not change throughout the universe. The charge on an electron, the speed of light in free space, the Planck constant, the gravitational constant, the electric constant, and the magnetic constant are all thought to be examples. It has been suggested that some fundamental constants might change with time, although there is no conclusive evidence that this occurs.
fundamental interactions The four different types of interaction that can occur between bodies. These interactions can take place even when the bodies are not in physical contact and together they
account for all the observed forces that occur in the universe. While the uniÜcation of these four types of interaction into a single theory has long been the aim of physicists, this has not yet been fully achieved. See also elementary particles; gauge theory; unified-field theory.
The gravitational interaction, some 1040 times weaker than the electromagnetic interaction, is the weakest of all. The force that it generates acts between all
bodies that have mass and the force is always attractive. The interaction can be visualized in terms of a classical *Üeld of force in which the strength of the force falls off with the square of the distance between the interacting bodies (see newton’s law of gravitation). The hypothetical gravitational quantum, the graviton, is also a useful concept in some contexts. On the atomic scale the gravitational force is negligibly weak, but on the cosmological scale, where masses are enormous, it is immensely important in holding the components of the universe together. Because gravitational interactions are long-ranged, there is a welldeÜned macroscopic theory in general relativity. At present, there is no satisfactory quantum theory of gravitational interaction.
The weak interaction, some 1010 times weaker than the electromagnetic interaction, occurs between *leptons and in the decay of hadrons. It is responsible for the *beta decay of particles and nuclei. In the current model, the weak interaction is visualized as a force mediated by the exchange of virtual particles, called intermediate vector bosons. The weak interactions are described by *electroweak theory, which uniÜes them with the electromagnetic interactions.
The electromagnetic interaction is responsible for the forces that control atomic structure, chemical reactions, and all electromagnetic phenomena. It accounts for the forces between charged particles, but unlike the gravitational interaction, can be either attractive or repulsive. Some neutral particles decay by electromagnetic interaction. The interaction is either visualized as a classical Üeld of force (see coulomb’s law) or as an exchange of virtual *photons. As with gravitational interactions, the fact that electromagnetic interactions are longranged means that they have a welldeÜned classical theory given by *Maxwell’s equations. The quantum theory of electromagnetic interactions is described by *quantum electrodynamics.
The strong interaction, some 102 times stronger than the electromagnetic interaction, functions only between *hadrons and is responsible for the force between nucleons that gives the atomic nucleus its
341 |
fuzzy logic |
great stability. It operates at very short range inside the nucleus (10–15 metre) and is visualized as an exchange of virtual mesons. The strong interactions are described by *quantum chromodynamics.
fundamental units A set of independently deÜned *units of measurement that forms the basis of a system of units. Such a set requires three mechanical units (usually of length, mass, and time) and, in some systems, one electrical unit; it has also been found convenient to treat certain other quantities as fundamental, even though they are not strictly independent. In the metric system the centi- metre–gram–second (c.g.s.) system was replaced by the metre–kilogram–second (m.k.s.) system; the latter now forms the basis for *SI units. In British Imperial units the foot–pound–second (f.p.s.) system was formerly used.
fungi A group of organisms comprising the kingdom Fungi. They can either exist as single cells or make up a multicellular body called a *mycelium, which consists of Ülaments known as *hyphae. Most fungal cells are multinucleate and have cell walls composed chieÛy of *chitin. Fungi exist primarily in damp situations on land and, because of the absence of chlorophyll, are either parasites or saprotrophs on other organisms. The principal criteria used in classiÜcation are the nature of the spores produced and the presence or absence of cross walls within the hyphae (see ascomycota; basidiomycota; deuteromycota; zygomycota). See also lichens.
fungicide See pesticide.
Fungi Imperfecti See deuteromycota.
funicle The stalk that attaches an ovule to the placenta in the ovary of a Ûowering plant. It contains a strand of conducting tissue leading from the placenta into the chalaza.
furan A colourless liquid compound, C4H4O; r.d. 0.94; m.p. –86°C; b.p. 31.4°C. It has a Üve-membered ring consisting of four CH2 groups and one oxygen atom.
furanose A *sugar having a Üvemembered ring containing four carbon atoms and one oxygen atom.
furfural A colourless liquid, C5H4O2, b.p. 162°C, which darkens on standing in air. It is the aldehyde derivative of *furan and occurs in various essential oils and in *fusel oil. It is used as a solvent for extracting mineral oils and natural resins and itself forms resins with some aromatic compounds.
fuse A length of thin wire made of tinned copper or a metal alloy of low
melting point that is designed to melt at a f speciÜed current loading in order to pro-
tect an electrical device or circuit from overloading. The wire is often enclosed in a small glass or ceramic cartridge with metal ends.
fused ring See ring.
fusel oil A mixture of high-molecular weight *alcohols containing also esters and fatty acids, sometimes formed as a toxic impurity in the distillation products of alcoholic fermentation. It is used as a source of higher alcohols and in making paints and plastics.
fusible alloys Alloys that melt at low temperature (around 100°C). They have a number of uses, including constanttemperature baths, pipe bending, and
automatic sprinklers to prevent Üres from spreading. Fusible alloys are usually *eutectic mixtures of bismuth, lead, tin, and cadmium. Wood’s metal and Lipowitz’s alloy are examples of alloys that melt at about 70°C.
fusion 1. Melting. 2. See nuclear fusion. 3. The combining together of cells, nuclei, or cytoplasm. See cell fusion; fertilization.
fusion reactor See thermonuclear reactor.
fuzzy logic A form of logic that allows for degrees of imprecision, used in *artiÜcial intelligence studies. More traditional logics deal with two truth values: ‘true’ and ‘false’. Fuzzy logics are multivalued dealing with concepts such as ‘fairly true’ and ‘more or less true’. These can be represented by numbers within a range [0,1], with the number representing the degree of truth. Fuzzy control applies fuzzy logic to the computer-control of processes.

G
GABA See gamma-aminobutyric acid.
gabbro A coarse-grained basic intrusive igneous rock with a similar composition to *basalt, i.e. mainly plagioclase feldspar, and pyroxine, with some olivine. Accessory minerals include apatite, hornblende, ilmenite, and magnetite.
Gabor, Dennis (1900–79) Hungarianborn British physicist, who worked as a research engineer from 1927 until 1933, when he joined the British ThomsonHouston company. In 1948 he joined the staff of Imperial College, London. In that same year, while working on electron microscopes, he invented *holography, for which he was awarded the 1971 Nobel Prize.
Gabriel reaction A method of making a primary *amine (free from any secondary or tertiary amine impurities) from a haloalkane (alkyl halide) using potassium phthalimide. It is named after Siegmund Gabriel (1851–1924).
gadolinium Symbol Gd. A soft silvery metallic element belonging to the *lanthanoids; a.n. 64; r.a.m. 157.25; r.d. 7.901 (20°C); m.p. 1313°C; b.p. 3266°C. It occurs in gadolinite, xenotime, monazite, and residues from uranium ores. There are seven stable natural isotopes and eleven artiÜcial isotopes are known. Two of the natural isotopes, gadolinium–155 and gadolinium–157, are the best neutron absorbers of all the elements. The metal has found limited applications in nuclear technology and in ferromagnetic alloys (with cobalt, copper, iron, and cerium). Gadolinium compounds are used in electronic components. The element was discovered by Jean de Marignac (1817–94) in 1880.
Gaia hypothesis The theory, based on an idea put forward by the British scientist James Ephraim Lovelock (1919– ), that the whole earth, including both its
biotic (living) and abiotic (nonliving) components, functions as a single selfregulating system. Named after the Greek earth goddess, it proposes that the responses of living organisms to environmental conditions ultimately bring about changes that make the earth better adapted to support life; the system would rid itself of any species that adversely affects the environment. The theory has found favour with many conservationists.
gain See amplifier.
galactic centre The region at the centre of a galaxy. In the Milky Way (our Galaxy) it corresponds to a radio source in the direction of Sagittarius. This source, called Sagittarius A, may correspond to a massive *black hole.
galactic merger The joining of two galaxies that approach within each other’s gravitational Üelds. They spiral together, forming one galaxy and producing a starburst, in which many new stars are created by the collapse of interstellar clouds. Streams of stars, some large enough to be dwarf galaxies, form tails behind the new galaxy.
galactic nucleus A bulge of older stars (population II, see population type) that surrounds the centre of a galaxy. The spiral arms of a spiral galaxy (such as the Milky Way) originate at the galactic nucleus.
galactose A simple sugar, C6H12O6, stereoisomeric with glucose, that occurs naturally as one of the products of the enzymic digestion of milk sugar (lactose) and as a constituent of gum arabic.
galactosidase See lactase.
galaxy A vast collection of stars, dust, and gas held together by the gravitational attraction between its components. Galaxies are usually classiÜed as elliptical, spiral, or irregular in shape. Elliptical galaxies appear like ellipsoidal clouds of
343 |
gall |
stars, with very little internal structure apart from (in some cases) a denser nucleus. Spiral galaxies are Ûat disc-shaped collections of stars with prominent spiral arms. Irregular galaxies have no apparent structure or shape.
The sun belongs to a spiral galaxy known as the Galaxy (with a capital G) or the Milky Way System. There are some 1011 stars in the system, which is about 30 000 parsecs across with a maximum thickness at the centre of about 4000 parsecs. The sun is about 10 000 parsecs from the centre of the Galaxy.
The galaxies are separated from each other by enormous distances, the nearest large galaxy to our own (the Andromeda galaxy) being about 6.7 × 105 parsecs away.
galaxy cluster A group of *galaxies containing many hundreds of members extending over a radius of up to a few megaparsecs (there also exist small groups of galaxies, such as the *Local Group, with a few tens of members). The richest and most regular clusters, such as the Coma cluster, with thousands of members, are gravitationally bound systems; it is not certain whether other less regular and less concentrated clusters are also bound. As well as galaxies, the clusters contain hot intracluster gas, at temperatures between 107 and 108 K; this can be detected by its X-ray emission. On a scale larger than clusters there are also superclusters, with extents of the order of a hundred megaparsecs, containing about
a hundred galaxies. It is not known whether superclusters are gravitationally bound. See also missing mass.
Galen (c. 130–c. 200) Greek physician, who studied in Pergamum, Corinth, and Alexandria. He practised in Pergamum before moving to Rome. Galen’s writings from this time became the basis of medical teaching and practice for 1500 years.
galena A mineral form of lead(II) sulphide, PbS, crystallizing in the cubic system; the chief ore of lead. It usually occurs as grey metallic cubes, frequently in association with silver, arsenic, copper, zinc, and antimony. Important deposits occur in Australia (at Broken Hill), Ger-
many, the USA (especially in Missouri, Kansas, and Oklahoma), and the UK.
Galilean satellites The four largest satellites (moons) of Jupiter, so-called because they were discovered and described by Galileo in 1610. They are, in order of increasing distance from the planet, Io, Europa, Ganymede and Callisto.
Galilean telescope See telescope.
Galilean transformations A set of equations for transforming the position and motion parameters from a frame of
reference with origin at O and coordinates g (x,y,z) to a frame with origin at O′ and coordinates at (x′,y′,z′). They are:
x′ = x – vt
y′ = y
z′ = z
t′ = t
The equations conform to Newtonian mechanics. Compare lorentz transformations.
Galileo Galilei (1564–1642) Italian astronomer and physicist. In 1583 he noticed that the time of swing of a *pendulum is independent of its amplitude, and three years later invented a hydrostatic balance for measuring *relative densities. He became a professor in Padua in 1592 and it was there (in 1610) that he made his Ürst astronomical telescope. With it he discovered four satellites of Jupiter, mountains on the moon, and sunspots. Returning to Pisa, his birthplace, he studied motion, demonstrating that the speed of a falling body is independent of its weight. He also gave open support to the sun-centred theory of the universe advocated by *Copernicus, a stand that brought him into conÛict with the church. He was summoned to Rome, forced to retract before the Inquisition, and banished under house arrest.
gall An abnormal growth of a plant tissue or organ elicited by a foreign organism. Galls most frequently occur as swellings or pits in stems, roots, leaves, and buds. Organisms responsible for their formation include bacteria, viruses, fungi, nematodes, mites, and insects. The gall structure is typically very distinct from

gall bladder |
344 |
surrounding normal tissue and often is characteristic of the eliciting organism. The mechanisms underlying gall formation are known in only a few cases. The bacterium *Agrobacterium tumefaciens, which is responsible for crown galls, induces a genetic change in infected host tissue by transfer of a plasmid bearing tumour-forming genes.
gall bladder A small pouch attached to the *bile duct, present in most vertebrates. *Bile, produced in the *liver, is
gstored in the gall bladder and released when food (especially fatty substances) enters the duodenum.
gallium Symbol Ga. A soft silvery metallic element belonging to group 13 (for-
merly IIIB) of the periodic table; a.n. 31; r.a.m. 69.72; r.d. 5.90 (20°C); m.p. 29.78°C; b.p. 2403°C. It occurs in zinc blende, bauxite, and kaolin, from which it can be extracted by fractional electrolysis. It also
occurs in gallite, CuGaS2, to an extent of 1%; although bauxite only contains 0.01% this is the only commercial source. The two stable isotopes are gallium–69 and gallium–71; there are eight radioactive isotopes, all with short half-lives. The metal has only a few minor uses (e.g. as an activator in luminous paints), but gallium arsenide is extensively used as a semiconductor in many applications. Gallium corrodes most other metals because it rapidly diffuses into their lattices. Most gallium(I) and some gallium(II) com-
pounds are unstable. The element was Ürst identiÜed by Paul Lecoq de Boisbaudran (1838–1912) in 1875.
gallon 1. (Imperial gallon) The volume occupied by exactly ten pounds of distilled water of density 0.998 859 gram per millilitre in air of density 0.001 217 gram per millilitre. 1 gallon = 4.546 09 litres (cubic decimetres). 2. A unit of volume in the US Customary system equal to
0.832 68 Imperial gallon, i.e. 3.785 44 litres.
GALP See glyceraldehyde 3-phosphate.
Galvani, Luigi (1737–98) Italian physiologist. In the late 1770s he observed that the muscles of a dead frog twitched when touched by two different metals. He concluded that the muscle was producing
electricity, later disproved by *Volta (who showed that the two metals and body Ûuids formed a battery). Galvani invented *galvanized iron and the *galvanometer.
galvanic cell See voltaic cell.
galvanized iron Iron or steel that has been coated with a layer of zinc to protect it from corrosion. Corrugated mild-steel sheets for rooÜng and mild-steel sheets for dustbins, etc., are usually galvanized by dipping them in molten zinc. The formation of a brittle zinc–iron alloy is prevented by the addition of small quantities of aluminium or magnesium. Wire is often galvanized by a cold electrolytic process as no alloy forms in this process. Galvanizing is an effective method of protecting steel because even if the surface is scratched, the zinc still protects the underlying metal. See sacrificial protection.
galvanometer An instrument for detecting and measuring small electric currents. In the moving-coil instrument a pivoted coil of Üne insulated copper wire surrounds a Üxed soft-iron core between the poles of a permanent magnet. The interaction between the Üeld of the permanent magnet and the sides of the coil, produced when a current Ûows through it, causes a torque on the coil. The moving coil carries either a pointer or a mirror that deÛects a light beam when it moves; the extent of the deÛection is a measure of the strength of the current. The galvanometer can be converted into an *ammeter or a *voltmeter. Digital electronic instruments are increasingly replacing the moving-coil type. See also ballistic galvanometer.
gametangium An organ that produces gametes. The term is usually restricted to the sex organs of algae, fungi, mosses, and ferns. See antheridium; archegonium; oogonium.
gamete A reproductive cell that fuses with another gamete to form a zygote. Examples of gametes are ova and spermatozoa. Gametes are *haploid, i.e. they contain half the normal (diploid) number of chromosomes; thus when two fuse, the diploid number is restored (see fertiliza-
345 |
gas |
tion). Gametes are formed by *meiosis.
See also sexual reproduction.
gametogenesis The processes involved in the formation of gametes. Gametes are normally formed by *meiosis but sometimes by *mitosis (as in the gametophyte generation of the ferns). In mammals gametogenesis in the female is known as *oogenesis and occurs in the ovaries; in the male it is known as *spermatogenesis and occurs in the testes.
gametophyte The generation in the life cycle of a plant that bears the gameteproducing sex organs. The gametophyte is *haploid. It is the dominant phase in the life cycle of mosses and liverworts, the *sporophyte generation depending on it either partially or completely. In clubmosses, horsetails, and ferns it is the *prothallus. In seed plants it is very much reduced. For example, in angiosperms the pollen grain is the male gametophyte and the embryo sac is the female gametophyte. See also alternation of genera-
tions.
gamma-aminobutyric acid (GABA)
An inhibitory *neurotransmitter in the central nervous system (principally the brain) that is capable of increasing the permeability of *postsynaptic membranes. GABA is synthesized by *decarboxylation of the amino acid glutamate.
gamma globulin See globulin.
gamma-iron See iron.
gamma radiation Electromagnetic radiation emitted by excited atomic nuclei during the process of passing to a lower excitation state. Gamma radiation ranges in energy from about 10–15 to 10–10 joule (10 keV to 10 MeV) corresponding to a wavelength range of about 10–10 to 10–14 metre. A common source of gamma radiation is cobalt–60, the decay process of which is:
6207 Co →β 6208 Ni →γ 6208 Ni
The de-excitation of nickel–60 is accompanied by the emission of gamma-ray photons having energies 1.17 MeV and 1.33 MeV.
gamma-ray astronomy *Astronomy involving gamma ray photons (with energies in excess of 100 MeV). The cosmic ra-
diation with the highest energy can be detected by electron–photon cascades, which take place in the atmosphere.
Gamma rays having lower energies can only be detected above the atmosphere. Many high-energy processes in *astrophysics are responsible for the production of gamma rays; one example is the decay of neutral *pions.
An interesting phenomenon is the gamma-ray burst. These events last for a few seconds, during which they are the strongest source of gamma rays in the
sky. It is thought that they may be the re- g sult of the formation of a *black hole, ei-
ther when a large star collapses or when two neutron stars collide.
ganglion A mass of nervous tissue containing many *cell bodies and *synapses, usually enclosed in a connective-tissue sheath. In vertebrates most ganglia occur outside the central nervous system; exceptions are the *basal ganglia in the brain. In invertebrates ganglia occur along the nerve cords and the most anterior pair (cerebral ganglia) are analogous to the vertebrate brain; invertebrate ganglia constitute a part of the central nervous system.
gangue Rock and other waste material present in an ore.
ganoid scale See scales.
garnet Any of a group of silicate minerals that conform to the general formula A3B2(SiO4)3. The elements representing A may include magnesium, calcium, manganese, and iron(II); those representing B may include aluminium, iron(III), chromium, or titanium. Six varieties of garnet are generally recognized:
pyrope, Mg3Al2Si3O12; almandine, Fe32+Al2Si3O12; spessartite, Mn3Al2Si3O12; grossularite, Ca3Al2Si3O12; andradite, Ca3(Fe3+,Ti)2Si3O12;
uvarovite, Ca3Cr2Si3O12.
Varieties of garnet are used as gemstones and abrasives.
gas A state of matter in which the matter concerned occupies the whole of its container irrespective of its quantity. In an *ideal gas, which obeys the *gas laws exactly, the molecules themselves would have a negligible volume and negligible
