
конъюгаты с фс
.pdf
REVIEW
pubs.acs.org/bc
The Controlled Display of Biomolecules on Nanoparticles: A Challenge Suited to Bioorthogonal Chemistry
W. Russ Algar,†,^ Duane E. Prasuhn,† Michael H. Stewart,‡ Travis L. Jennings,§ Juan B. Blanco-Canosa,|| Philip E. Dawson,|| and Igor L. Medintz*,†
†Center for Bio/Molecular Science and Engineering, Code 6900, ‡Optical Sciences Division, Code 5611, U.S. Naval Research Laboratory, 4555 Overlook Avenue, S.W., Washington, DC 20375, United States
§eBioscience, Inc., 10255 Science Center Drive, San Diego, California 92121, United States
)
Departments of Chemistry and Cell Biology, The Scripps Research Institute, La Jolla, California 92037, United States
^College of Science, George Mason University, 4400 University Drive, Fairfax, Virginia 22030, United States
ABSTRACT: Interest in developing diverse nanoparticle (NP)- biological composite materials continues to grow almost unabated. This is motivated primarily by the desire to simultaneously exploit the properties of both NP and biological components in new hybrid devices or materials that can be applied in areas ranging from energy harvesting and nanoscale electronics to biomedical diagnostics. The utility and e ectiveness of these composites will be predicated on the ability to assemble these structures with control over NP/biomolecule ratio, biomolecular orientation, biomolecular activity, and the separation distance within the NPbioconjugate architecture. This degree of control will be especially critical in creating theranostic NP-bioconjugates that, as a single vector, are capable of multiple functions in vivo, including target-
ing, image contrast, biosensing, and drug delivery. In this review, a perspective is given on current and developing chemistries that can provide improved control in the preparation of NP-bioconjugates. The nanoscale properties intrinsic to several prominent NP materials are briefly described to highlight the motivation behind their use. NP materials of interest include quantum dots, carbon nanotubes, viral capsids, liposomes, and NPs composed of gold, lanthanides, silica, polymers, or magnetic materials. This review includes a critical discussion on the design considerations for NP-bioconjugates and the unique challenges associated with chemistry at the biological nanoscale interface—the liabilities of traditional bioconjugation chemistries being particularly prominent therein. Select bioorthogonal chemistries that can address these challenges are reviewed in detail, and include chemoselective ligations (e.g., hydrazone and Staudinger ligation), cycloaddition reactions in click chemistry (e.g., azide alkyne cyclyoaddition, tetrazine ligation), metal-a nity coordination (e.g., polyhistidine), enzyme driven modifications (e.g., HaloTag, biotin ligase), and other sitespecific chemistries. The benefits and liabilities of particular chemistries are discussed by highlighting relevant NP-bioconjugation examples from the literature. Potential chemistries that have not yet been applied to NPs are also discussed, and an outlook on future developments in this field is given.
’INTRODUCTION
Nanoparticles (NPs) are materials with dimensions that are typically less than 100 nm. In many cases, NPs are synthetic colloids prepared from metals, alloys, semiconductors, carbon allotropes, or polymers. Other NP materials are biologically derived and include bacteriophages and other viral particles, liposomes, or biopolymers such as polysaccharides. The interest in NP materials is as diverse as the range of materials, with target applications in biology and medicine, catalysis, energy, electronics, and computing, to name only a few. In particular, the application of NPs in biological diagnostics, imaging, and therapeutics is an active area of research necessitating highly interdisciplinary e orts that combine materials science with expertise in biology and medicine. The ability to prepare NP-bioconjugates
in a controlled manner is fundamental to these research e orts and is the focus of this review.
While NPs have their own intrinsic properties, it is generally necessary in biological applications to impart additional properties or functions through physical or chemical coupling between a NP and one or more molecules. For biologically derived NPs, a common example is labeling with a fluorescent dye or another type of reporter molecule to enable tracking and detection. The converse is often true in the case of many synthetic materials, where the NP enables detection but requires conjugation to a
Received: February 1, 2011
Revised: March 15, 2011
Published: May 18, 2011
r2011 American Chemical Society |
825 |
dx.doi.org/10.1021/bc200065z | Bioconjugate Chem. 2011, 22, 825–858 |

Bioconjugate Chemistry |
|
REVIEW |
biological moiety for targeting or bioactivity. The association of one or more biologically relevant molecules at the interface of a NP defines a NP-bioconjugate, and combines the unique optoelectronic or physicochemical properties of NP materials with biological activity such as selective binding. Biomolecules of interest may include one or more of the following:
•Peptides, proteins, and antibodies
•Enzymes and ribozymes
•Oligonucleotides and aptamers
•Carbohydrates
•Lipids
•Drugs or other biologically active small molecules
•Reporter molecules or contrast agents (e.g., radiolabels, fluorescent dyes)
Analogous to traditional protein labeling chemistry, NP-bio- conjugates can be prepared via the formation of new chemical bonds between functional groups associated with a NP and the biomolecule or small molecule of interest. NPs may also o er the potential for association through dative or coordinate bonding, electrostatic interactions, and van der Waals interactions. Moreover, it can often be necessary to account for a second conjugate preparation, where the biomolecule of interest is also conjugated with a reporter molecule. This is frequently the case in biosensing applications that use NPs. In general, the structure of a NP-bioconjugate a ects its function, and the controlled display of biomolecules on NPs is paramount in obtaining conjugates with well-defined and reproducible properties.
To date, researchers have largely relied upon the traditional chemistries associated with protein labeling for the preparation of NP-bioconjugates. However, the range of bioconjugate chemistries used with NPs has lagged behind the multitude of biological applications proposed. Although traditional bioconjugate chemistries have been adequate for proof-of-concept studies, the optimization of NP-bioconjugates for real applications (e.g., clinical) will require much greater control than these chemistries can o er. “Shotgun” or heterogeneous approaches for the preparation of NP-bioconjugates are rarely suitable for maximizing the potential of NPs in biological applications. Rather, clean, e cient, and bioorthogonal conjugation reactions are required to eliminate undesirable side reactions, minimize nonspecific NP-bioconjugate activity, improve reproducibility in production, and maximize e cacy. This review highlights the emergence of novel approaches for the preparation of NPbioconjugates that target these goals. The following sections highlight the properties of di erent NP materials and provide examples of their application as NP-bioconjugates. The ideals and challenges associated with NP-bioconjugates are discussed along with bioconjugation chemistry derived from chemoselective ligations, cycloaddition reactions, metal-a nity coordination, and enzymatic labeling in the context of their application to NPs. The reader should note that references to “standard bioconjugate chemistry” or “standard techniques” imply the family of currently used chemistries that were developed for modifying biomolecules with fluorophores and other labels. These are the techniques that are described extensively in Hermanson’s Bioconjugate Techniques and Haugland’s The Handbook: A Guide to Fluorescent Probes and Labeling Technologies.1,2
Carbodiimide chemistry for coupling amines and carboxyls, succinimidyl (NHS) ester, or isothiocyanate targeting of primary amines, and maleimide reactions with thiols are typical examples.
’NANOPARTICLE MATERIALS, PROPERTIES, AND APPLICATIONS
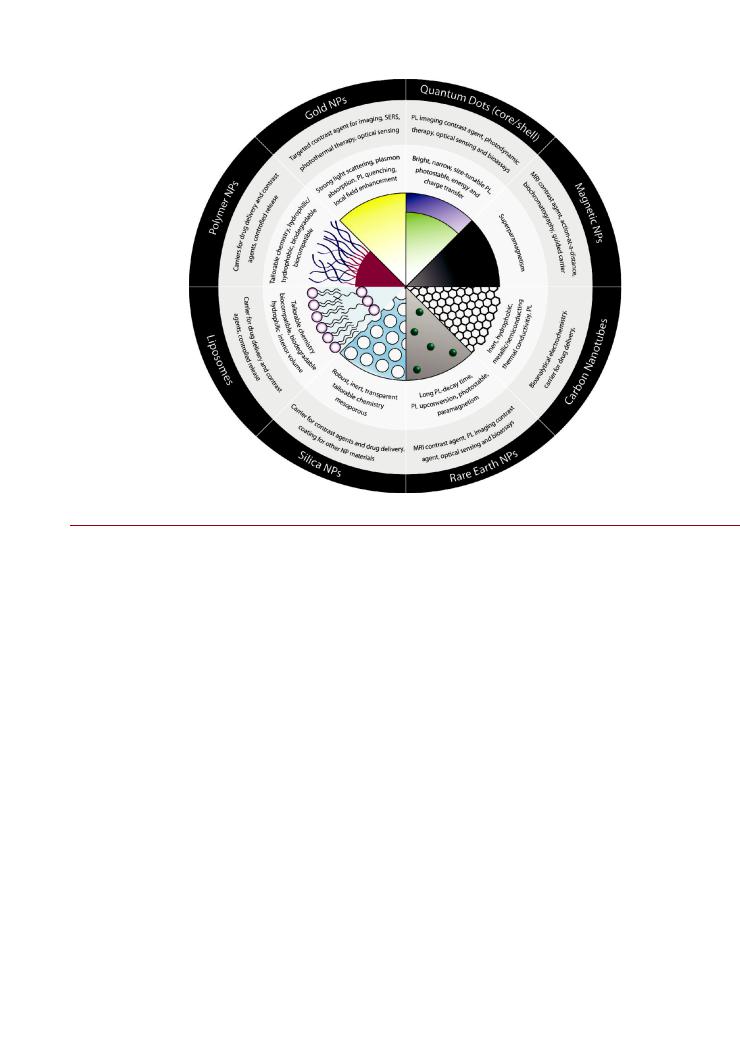
This section provides a brief overview of the NPs commonly used in biological applications: the di erent materials, their unique properties at the nanoscale, and the general features of their surface chemistry. The interested reader can find further details in the cited references, and should note that the list of materials and coatings described here is far from comprehensive. Figure 1 presents a summary of select NP materials and properties.
Gold and Silver. Gold NPs (Au NPs) typically range in size from 1 to 100 nm and are characterized by strong optical absorption and light scattering. These optical properties arise from the phenomenon of localized surface plasmon resonance (LSPR), wherein the conduction band electrons of an Au NP oscillate collectively and in resonance with certain wavelengths of incident light.3 5 Solid spherical Au NPs exhibit a weakly sizedependent LSPR band in their extinction spectra that is located in the visible region of the spectrum. Rod-shaped Au NPs exhibit two LSPR bands that correspond to transverse and longitudinal oscillations of electrons. The longitudinal resonance mode is located in the near-infrared region of the spectrum, and is highly tunable by changing the aspect ratio of the nanorod. Hollow or dielectric-filled Au NPs are referred to as gold nanoshells and also exhibit a strong tunable LSPR mode that depends on the thickness of the shell. The wavelength-dependent intensity of the LSPR is very sensitive to changes in the dielectric environment surrounding the Au NP, as well as plasmonic coupling between NPs, and this has provided a mechanism for biological sensing based on colorimetric changes that result from selective binding interactions at the surface of Au NPs.6 The ability of Au NPs to efficiently quench fluorescence has also been used to
develop sensing methods.7 Recent reviews have highlighted these applications.3,4,8,9 The LSPR effect in Au NPs can also
amplify Raman scattering due to local electric field enhancement at the NP during optical interrogation and provides another modality for sensing.10,11 In imaging applications, Au NPs are potentially a very sensitive probe that can provide elastically
scattered light intensities that are orders of magnitude larger than the fluorescence emission of dyes.3,4,12 Furthermore, the absorp-
tion of light by Au NPs and the rapid thermalization of that energy are ideal for use in photothermal therapy.3,4,13,14 Silver
NPs (Ag NPs), Au Ag alloyed NPs, and Au/Ag core/shell NPs are emerging as an alternative to Au NPs.5 The LSPR effect with Ag NPs offers a narrower resonance, stronger elastic light scattering, and greater enhancement of Raman scattering potentially providing better sensitivity than Au NPs for some biological applications. Fluorescent Au and Ag NPs have been synthesized,15 although their applications have not been widely characterized. Ag NPs also have strong antimicrobial properties that are being considered for use in medical applications.16
A wide array of synthetic methods for preparing Au and Ag NPs have been reported and are reviewed elsewhere.17 22 The
predominant chemistry for modifying Au NPs is the self-assem- bly of thiols via chemisorption.3 5,12,23 Small molecules, poly-
mers, and a variety of biomolecules can be anchored to Au NPs through thiol-terminated linkers. Bifunctional thiol ligands that display, for example, carboxyl or amine groups also enable further modifications using standard techniques to prepare Au NPbioconjugates. The chemisorption of thiols on silver24 can also be applied to the preparation of Ag NP-bioconjugates.25
826 |
dx.doi.org/10.1021/bc200065z |Bioconjugate Chem. 2011, 22, 825–858 |
Bioconjugate Chemistry |
|
REVIEW |
Figure 1. Select nanoparticle (NP) materials, their properties of interest, and prominent biological applications.
Semiconductors. Colloidal quantum dots (QDs) are luminescent crystalline NPs composed of semiconductor materials with radii that are typically between 2 and 10 nm.26 28 Many
different II VI or III V semiconductors,29,30 alloys thereof,31 and group IV semiconductors32,33 have been used to synthesize
colloidal QDs. In bioapplications, the interest has been in materials that offer visible or near-infrared photoluminescence (PL). The most widely used materials are CdX/ZnS QDs (X = Se, Te), where a CdX core is overcoated with a few atomic layers of wider bandgap ZnS or other binary semiconductor materials to improve its luminescence properties.34,35 The optical properties of QDs are much different than their bulk analogues and arise from the quantum confinement of charge carriers within the dimensionality of the nanocrystal.36 39 High-quality QDs exhibit a narrow PL band that can be tuned across a region of the visible or near-infrared spectrum by synthetically growing different nanocrystal sizes; the limits of the tunable emission range are determined by the choice of material.26 28 Furthermore, the PL emission of QDs is more photostable than that of organic dye molecules, QDs offer stronger oneand two-photon absorption across a broader spectral range, and—similar to dye molecules— QDs can participate in energy transfer mechanisms that can modulate PL. The interest in QDs for biological applications is rooted in these more favorable optical properties. Several reviews have highlighted the use of QDs as probes for cellular, tissue, or in vivo imaging,40 and in biological sensing.41,42 Despite the success of CdX/ZnS QDs, there is a current trend toward developing QD materials that do not incorporate heavy metals and offer deep red
or near-infrared PL at small nanocrystal dimensions. Examples include InP and CuInSe as core materials.43,44 Quantum rods
have also been developed and are essentially elongated QDs with a nonunity aspect ratio.45,46 The optical properties of quantum rods are not dissimilar to those of QDs, but do exhibit some unique features such as polarized emission.
As synthesized with native alkyl coordinating ligands, II VI QDs such as CdSe/ZnS are hydrophobic and insoluble in aqueous media. The two most common strategies for imparting
aqueous solubility are the use of bifunctional ligand coatings and bifunctional polymer coatings.26,28,47 The former coordinate to
the QD surface through a chemical function (e.g., thiol, imidazole) and replace the native hydrophobic ligands; the latter have pendant alkyl chains that interdigitate with the native ligands via hydrophobic interactions. In both cases, a second and polar chemical function—such as a carboxylate, amine, or poly(ethylene glycol) (PEG) chain—mediates aqueous solubility. Mixtures of di erent ligands and the use of copolymers enable the modification of QDs with multiple functional groups. Ligand coatings are advantageous in that more compact QDs are obtained;48 however, polymer coated QDs tend to have superior brightness and photostability. Standard bioconjugate techniques, such as carbodiimide coupling, are widely used with both ligand and polymer stabilized QDs. Ligand coatings have also frequently enabled the preparation of bioconjugates through selfassembly driven by coordination to the QD surface. Typical coating and bioconjugate methods for quantum rods are analogous to those for QDs.
Magnetic Materials. Magnetic NPs are typically less than 20 nm in diameter and have been prepared from different materials, including Co, Fe, Mn, Ni, γ-Fe2O3, Fe3O4, FePt, and other oxides or alloys.49 56 Magnetic NPs are interesting due to
827 |
dx.doi.org/10.1021/bc200065z |Bioconjugate Chem. 2011, 22, 825–858 |

Bioconjugate Chemistry |
|
REVIEW |
the combination of physical size and colloidal dispersion, the capacity for “action at distance” using magnetic fields, and their effectiveness as negative contrast agents in T2 weighted magnetic resonance imaging (MRI).57,58 In addition to MRI imaging, magnetic NPs have potential as concurrent drug delivery vehicles or therapeutic agents in hyperthermia treatments with the added possibility of magnetic targeting. Magnetic NPs are also useful in bioaffinity chromatography methods (i.e., magnetic capture) for sample purification, analyte preconcentration, or toxin decor-
poration. These diverse applications are discussed in several reviews.54,59 62 There has also been a recent trend toward the
synthesis of “dumbbell” magnetic NPs that are inorganically linked dimers of a magnetic NP and a second class of NP. Most commonly, the latter is an optically active NP material such as gold or CdS63,64 that allows the combination of optical tracking with the functionality of magnetic NPs. In addition to dumbbell NPs, analogous core/shell structures are also possible.64
Most synthetic methods yield hydrophobic magnetic NPs that are unsuitable for direct biological application. Similar to QD synthesis, the native ligands can be exchanged with bifunctional ligands or the NPs coated with polymer molecules. Functional groups that can bind to the surface of many magnetic NP materials include carboxylic acids, catechols, phosphates, sulfates, and amines. The groups that mediate aqueous solubility can often be used for subsequent bioconjugation using standard techniques. A second strategy commonly used with magnetic NPs is to coat them with an inorganic material such as silica, which can provide a hydrophilic protective layer against oxidation. The wide range of chemistry available to tailor the surface functionality of silica (vide infra) provides flexibility and the opportunity for bioconjugation in this approach.
Carbon Allotropes. Several different types of NP are derived from carbon allotropes. Carbon nanotubes (CNTs) are the most well-known of these materials, and are sheets of graphene rolled up into a seamless tube. If the nanotube is composed of a single graphene sheet, it is referred to as a single-walled carbon nanotube (SWCNT). In contrast, if it is composed of multiple sheets of graphene rolled concentrically, it is called a multiwalled carbon nanotube (MWCNT)—essentially a Russian doll of SWCNTs. Typical diameters of SWCNTs are 0.3 3.0 nm, whereas the diameter of MWCNTs can reach more than 100 nm. CNT lengths can vary from nanometers to micrometers. In bioapplications, it is the electronic and optical properties of CNTs combined with their large surface-to-volume ratios which are of interest.65,66 A SWCNT can be metallic or semiconducting depending on its roll-up vector. The size of the band gap in chiral SWCNTs is dependent on the nanotube diameter, and photostable near-infrared PL can be associated with the band gap. Recent reviews have detailed the special properties and biological applications of CNTs,67,68 including applications in electrochemical and electro-optical biosensing,69 71 and as cellular delivery vectors and imaging probes.72 74
Similar to CNTs, fullerenes are composed of a graphene sheet, but are wrapped up into a closed, approximately spherical polyhedron rather than a tube. This geometry arises through the incorporation of five-membered rings into the otherwise sixmembered ring structure of graphene. Buckministerfullerene, C60, is the most well-known example; however, C70 and other variants are also common. Fullerenes are interesting due to their photochemical activity (e.g., singlet oxygen production and DNA cleavage), their redox properties (e.g., radical scavenging), hydrophobicity, and electrophilicity (e.g., enzyme inhibition).
The interested reader is referred to several reviews on the biological application of fullerenes.75 77
Both CNTs and fullerenes are extremely hydrophobic and poorly soluble in aqueous media; therefore, additional functionalization is required for most bioapplications. Two general strategies are utilized in this respect: the adsorption or complexation of macromolecules and surfactants, or alternatively, covalent modification of the carbon NP. For example, cyclodextrins or polyvinylpyrrolidone can be used to coat and solubilize C60, while cycloaddition reactions are widely used to introduce new functional groups.77 In the case of CNTs, a bifunctional pyrene molecule (e.g., 1-pyrenebutanoic acid succinimidyl ester) can irreversibly adsorb through π-stacking interactions and be used as a reactive linker for bioconjugation.78,79 The method most commonly used for the covalent modification of CNTs is oxidation under acidic conditions, where carboxyl groups are introduced selectively at the highly curved tips of the CNT.65,80 Modification of the sidewall is usually less e cient, and largely limited to defect sites. The introduction of carboxyl groups can enable many standard bioconjugate techniques including carbodiimide coupling. However, the drawback to this approach is a degradation of the electronic properties of the CNT due to the loss of π-conjugation at reaction sites. Further details on the chemistry of CNTs—including covalent modification schemes
such as cycloaddition and reactivity with diazonium salts—can be found in reviews written by Tasis and co-workers.81,82
Other carbon allotropes have recently emerged as biologically
useful nanomaterials, including two-dimensional graphene and graphene oxide sheets,83,84 carbon quantum dots, as well as
nanodiamonds.85 In contrast to CNTs and fullerenes, nanodiamonds are not based on the sp2-hybridized carbon centers of graphene. Rather, nanodiamonds are a lattice of tetrahedral sp3- hybridized carbon centers, and exhibit visible and photostable PL due to the presence of defect sites.86 The surface of nanodiamonds has intrinsic reactivity, and many synthetic preparations yield an interfacial layer of oxidized carbon groups. Carboxylated nanodiamonds are compatible with many standard bioconjugate techniques, are economical to synthesize, and are being explored as fluorescent cellular probes.86
Rare Earth Metals. The use of trivalent lanthanide ions in biology is well established. For example, Gd3þ is used as a contrast agent in MRI,87 while Eu3þ and Tb3þ are used in timeresolved fluorescence immunoassays.88 Historically, the properties of these ions have been harnessed in the form of chelates; however, incorporation of lanthanides into NPs provides many new opportunities and advantages.89,90 Lanthanide ions have visible or near-infrared PL with narrow spectral bandwidth, excellent photostability, and decay times that are 103 106-fold longer than other luminophores.89,90 The excitation of visible PL is possible using both ultraviolet and near-infrared light, where the latter is possible through the efficient luminescence upconversion exhibited by Er3þ, Tm3þ, and Ho3þ ions.91 93 Although individual lanthanide ions tend to be weak absorbers, localizing multiple ions in a NP host can increase the effective absorption cross section. In the case of upconversion, more strongly absorbing lanthanide ions such as Yb3þ can be co-doped into the NP host as sensitizers that absorb NIR light and transfer the excitation energy to the emitter or activator ions (e.g., Er3þ).91 93 In MRI, Gd3þ creates positive contrast in T1-weighted imaging through paramagnetic interactions with water. NPs provide the opportunity to concentrate multiple Gd3þ ions in small volume but retain a large surface area-to-volume ratio to promote
828 |
dx.doi.org/10.1021/bc200065z |Bioconjugate Chem. 2011, 22, 825–858 |

Bioconjugate Chemistry |
|
REVIEW |
interactions between Gd3þ and water. Further optimization of MRI contrast is possible through the slower rotational diffusion of NPs compared to chelates and the ability to use NP surface chemistry to maximize the water exchange rate.94
Lanthanide NPs are typically composed of a lanthanide oxide
(e.g., Y2O3), phosphate (e.g., LaPO4), or (sodium) fluoride (e.g., LaF3, LaNaF4).91,92,95 Most lanthanide ions easily substitute for
another in a host lattice. In optical applications, luminescent dopant ions are typically incorporated at not more than a few
percent. Sensitizer doping can be more substantial, on the order of 20%.91,92 In the case of magnetic resonance applications, a
Gd3þ-based NP material (e.g., GdF3, Gd2O3) is generally used, and other lanthanide ion dopants can add luminescence functionality.
Lanthanide NPs can be prepared by either aqueous or organic solvent routes,92,96,97 where the latter require further surface
modification for use in biological applications. In either case, aqueous lanthanide NPs are usually stabilized by surfactant ligands or polymers with carboxylate or phosphate groups that coordinate to the NP surface and also potentiate bioconjugation. A coating of hydrophilic silica can also be prepared around a lanthanide NP.98 This provides a robust and hydrophilic coating, along with the opportunity for diverse surface modification and bioconjugation (vide infra).
Silica. Silica is a robust, hydrophilic, and biocompatible material that can be readily modified with diverse chemical func-
tionality. As a consequence, silica is widely used as an inorganic coating for NPs of different composition (e.g., QDs,99,100 magnetic NPs,101 103 lanthanide NPs,98 and Au NPs104), or as a NP-based
carrier of functional molecules.105 Using the diversity of silane chemistry,106 a silica shell or coating can be tailored to have functional groups that can include, but are not limited to, amine, aldehyde, carboxyl, epoxy, and thiol groups. In addition to the use of silica as a structural shell on other NP materials, NPs composed of silica can be prepared with sizes that typically range between tens and hundreds of nanometers. The St€ober or reverse microemulsion methods are most commonly used for the synthesis of silica NPs.107 In bioapplications, the ability to dope silica NPs with another material is of great utility. For example, up to 104 dye molecules can be incorporated into silica NPs (ca. 50 75 nm diameter) during synthesis.108 The photostability of the dyes is greatly enhanced within the silica NP matrix, and the concentration of thousands of dye molecules into a single entity provides ultrabright labels for fluorescence imaging and assay
applications. In addition, silica NPs can be used as carriers in drug delivery.109,110 In this case, drug molecules must not be
permanently incorporated within the NPs. The addition of surfactants and other manipulations of reaction conditions during silica NP synthesis can yield mesoporous silica NPs with pore sizes as small as 1 nm or as large as many tens of nanometers. These nanoscale mesopores allow both cargo transport and release and are therefore attractive for drug delivery applications. Typical values for pore surface area and volumes are close to 103 m2 g 1 and 2 cm3 g 1, respectively,
thereby offering small molecule (e.g., drug) cargo capacities on the order of 101 102 mg g 1.110
Polymer and Amphiphile Nanoparticles. Polymer and other amphiphile-based NPs are currently the most prominent materials being utilized as nanoscale drug carriers. These include: polymeric NPs, dendrimers, liposomes, polymersomes, and micelles.111 114 The interest in these materials arises from the combination of nanoscale size with the nearly infinite diversity of physical properties and chemical functionality that can be
obtained through organic chemistry. Polymer and amphiphile NPs can be designed to do the following:
•Carry molecular cargo externally or internally
•Carry hydrophilic or hydrophobic cargo
•Release cargo gradually
•Exhibit “smart” physicochemical responses to environmental stimuli (e.g., pH, thermal response) to selectively release cargo
•Evade the reticuloendothelial system and other immune responses
•Biodegrade
•Target di erent tissues or cell types
These di erent properties are tailored through the selection of the chemical composition of the polymer NPs. Bioconjugates of polymer and amphiphile NPs are typically prepared to assist targeting— antibody conjugates being particularly common. While there is no characteristic surface chemistry due to the diversity of materials, the introduction of carboxyl or amine groups into the polymer/ amphiphile composition for purposes of bioconjugation is routine. Overall, the bioconjugation chemistry of these NPs is generally dictated by the functional groups associated with the material(s).
Polymer NPs are typically agglomerates of polymer chains with dimensionality that can range across the nanoscale. For example, poly(lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA) derivatives are commonly used to prepare NPs and are advantageous due to their biodegradability.115 Dendrimers are fractallike, hyperbranched polymers that can be size-controlled through their stepwise growth. A common example is polyamidoamine
dendrimers, which form spherical NPs and display a high density of surface amine groups.116,117 Liposomes and polymersomes are
spherical NPs with unilamellar or multilamellar structures that separate and encapsulate an aqueous interior from bulk aqueous solvent. The lamellae of liposomes and polymersomes are composed of a bilayer of either lipids or synthetic block copolymer amphiphiles, respectively, with a hydrophobic midplane to separate the two aqueous volumes. Distearoylphosphatidylcholine is an example of a lipid material commonly used for assembling liposomes. In contrast to liposomes, micelles consist of amphiphiles or surfactants that assemble into spherical assemblies with a hydrophobic interior that excludes aqueous solvent and is thus able to carry hydrophobic molecular cargo. Micelles are typically
prepared using the same types of amphiphiles as polymersomes— PEG-PLA is a common example.113
Viral Particles. Plant virus and bacteriophage capsids are natural examples of protein NPs. In nature, viruses are designed to carry genetic cargo, penetrate cells, and release their cargo—a natural parallel with the target application of many polymer and amphiphile-based NPs. Viral capsids are thus emerging as potential vehicles for drug delivery and contrast agents.118 121 The unique advantage of such materials is that, in contrast to many synthetic NP materials, there is almost no polydispersity in their size and morphology. Being composed of many identical protein subunits, viral capsids offer a well-defined and regular array of amino acid residues for chemical modification or bioconjugation at both their interior and exterior surfaces. They are also amenable to recombinant modification allowing site-specific placement of unique groups such as cysteine-thiols where needed.
The MS2 bacteriophage and cowpea mosaic virus (CPMV) capsids have dimensions of approximately 27 and 28 nm,122,123
respectively, and are two prominent viral NP candidates being prototyped for drug delivery and other bioconjugate applications.124
829 |
dx.doi.org/10.1021/bc200065z |Bioconjugate Chem. 2011, 22, 825–858 |
Bioconjugate Chemistry |
|
REVIEW |
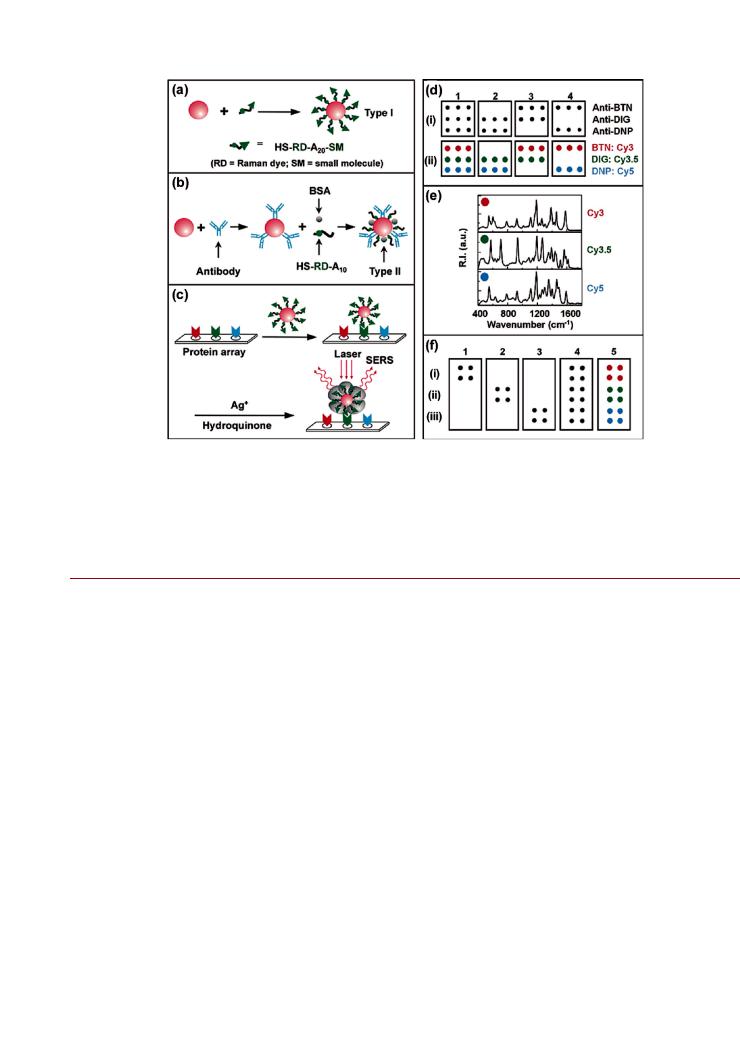
Figure 2. Protein microarray design using Au NPs. (a) Au NPs self-assembled with thiol-terminated oligonucleotides that are modified with a Raman active dye at the proximal terminus and small molecule probe at the distal terminus. (b) Au NPs modified with antibody probes and coated with Raman dye-modified oligonucleotides and bovine serum albumin (BSA). In panel and b, A20 and A10 refer to the number of adenine repeats. (c) Protein microarray experiments, showing the immobilization of proteins, selective labeling with Au NPs, and silver staining/enhancement. (d) Four di erent protein small molecule binding experiments (1 4) with (i) flatbed scanner images and (ii) color codes for the Raman spectra associated with the probes in the corresponding spots (BTN = biotin; DIG = digoxigenin; DNP = dinitrophenyl). (e) Raman spectra for the color coded spots in (d). (f) Four di erent protein protein binding experiments (1 4), and color codes for Raman identification (5), with flatbed scanner images for (i) Cy3-AuNP-antimouse IgG, (ii) Cy3.5-Au NP-antiubiquitin, and (iii) Cy5-Au NP-antihuman protein C. Figure adapted with permission from ref 125. Copyright 2003 American Chemical Society.
’REPRESENTATIVE APPLICATIONS OF NANOPARTICLE BIOCONJUGATES
A comprehensive summary of NP-bioconjugate applications is beyond the scope of this review. Only a few select examples of their application are given herein to illustrate the utility of NPbioconjugates.
Diagnostic Assay. The properties of optically active NPs can enable new possibilities in biological detection with high sensitivity. For example, Cao et al. developed a solid-phase hybridization assay10 and protein microarray125 (Figure 2) using Au/Ag NPs conjugated with dye-labeled oligonucleotides as reporters. The surface enhanced Raman scattering spectra of the dye on the NP provided both an analytical signal and analyte-specific fingerprint. In the case of the hybridization assay, detection was possible down to 20 fM. As described by Faulds et al., the
narrow features of Raman spectra can enable multiplexed detection without the spatial registration of capture probes.126,127 This
is an example of a new method that exploits the special properties of a NP and relies on the preparation of a NP-bioconjugate to harness those properties.
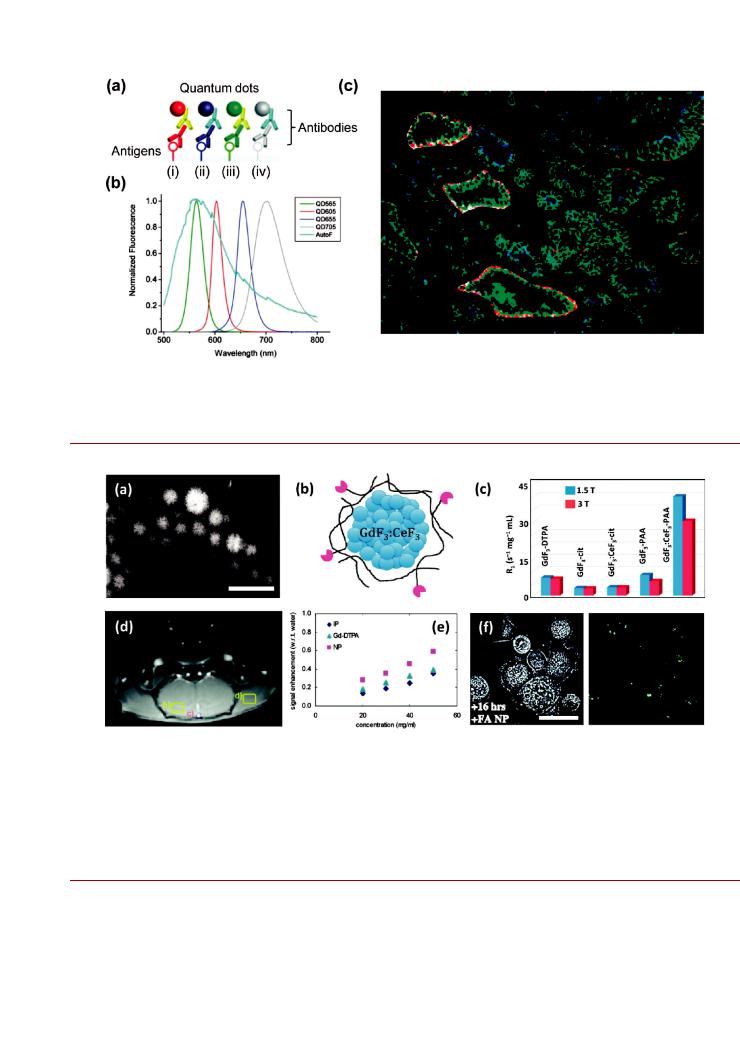
Targeted Contrast Agent. Optically active NPs provide advantages in diagnostic imaging, particularly in terms of sensitivity and facilitating improved multicolor analyses. Liu et al. used QD-antibody conjugates that targeted four different protein biomarkers in histological tissue specimens from prostatectomy
procedures (Figure 3).128 Multispectral imaging of the heterogeneous fluorescence staining pattern enabled the observation of single malignant cells and progressive cancer development within the complex microenvironment of the histological specimens. These results are an example of how the uniquely advantageous properties of a NP can facilitate advances in an established technique such as immunohistochemical staining. Although not explored in this example, the large two-photon cross section of QDs could potentially be exploited—in addition to advantages in brightness and multiplexing—to suppress tissue autofluorescence for far more sensitive immunohistochemical detection.
Multimodal Contrast Agent. Different medical imaging techniques have different capabilities for spatial and temporal resolution, tissue penetration, and contrast generation. A single probe that could provide contrast in different imaging modalities would be highly advantageous in diagnostics. NPs provide the opportunity to incorporate multiple materials into a single probe to enable multiple imaging modalities and thus allow for better overall contrast or resolution. Cheung et al. explored the use of different formulations of soluble Gd3þ-rich and Tb3þ (or Eu3þ) doped lanthanide fluoride NP aggregates for multimodal imaging (Figure 4).94 The NPs provided contrast enhancement in MRI imaging that was sufficient to enable animal perfusion imaging at NP doses that were 10-fold smaller than those associated with standard Gd3þ chelates. In addition, at the X-ray energies
830 |
dx.doi.org/10.1021/bc200065z |Bioconjugate Chem. 2011, 22, 825–858 |
Bioconjugate Chemistry |
|
REVIEW |
Figure 3. Multiplexed fluorescence immunostaining of prostate tissue specimens using QD-antibody conjugates. (a) Primary antibodies targeted tissue antigens and were labeled with secondary antibody-QD conjugates (i iv). As shown in (b), the QD emission peaks were at (i) 605, (ii) 655, (iii) 565, and (iv) 705 nm. The tissue autofluorescence (AutoF) background is shown for reference. (c) Processed fluorescence images highlighting four di erent protein biomarkers using the four colors of QD, shown in blue, green, red, and white pseudocolors. The staining pattern can di erentiate between healthy and cancerous tissue. Figure adapted with permission from ref 128. Copyright 2010 American Chemical Society.
Figure 4. Potential for multimodal NPs. (a) STEM image of GdF3:CeF3 NP aggregates stabilized with poly(acrylic acid) (PAA). The scale bar is 150 nm. (b) Pictorial representation of GdFe3:CeFe3 NP aggregates stabilized with PAA. The mean aggregate size is ca. 70 nm, while the fundamental unit size is estimated to be 10 12 nm. (c) Mass relaxivities (R1) for aqueous dispersions of di erent NP formulations. The GdF3:CeF3 NP aggregates have a much larger relaxivity than the commonly used Gd3þ-diethylenetriaminepentaacetic acid (DTPA) chelate. (d) Dynamic contrast enhancement MRI image of a rat brain using GdF3:CeF3 NP aggregates as a perfusion contrast agent. (e) Comparison of X-ray (25 kV) image signals as a function of mass concentration for aqueous PAA-stabilized NaGdF4:Eu3þ NP aggregates and the commonly used contrast agents Gd3þ-DTPA and iopromide (IP). The NPA aggregates provide superior contrast. (f) Transmitted light (left) and confocal fluorescence (right) images of SK-BR-3 cells showing uptake of folate (FA)-modified NaGdF4:Tb3þ NP aggregates. The scale bar is 20 μm. The punctate pattern of green emission in the fluorescence image is characteristic of endosomal uptake, and comparison with the transmitted light image indicates that the NPs are located within the cells. Figure adapted with permission from ref 94. Copyright 2010 American Chemical Society.
associated with mammography, the NPs were shown to have greater X-ray attenuation than the standard iopromide contrast agent used in computed tomography (CT). Luminescence from Tb3þ also provided the opportunity for fluorescence imaging of SK-BR-3 breast cancer cells using folate-conjugated NPs that promoted cellular uptake.94 Although a single NP vector has not
yet been fully developed to provide these three contrast mechanisms concurrently, this example makes clear the long-term potential for NP materials as multimodal contrast agents using a combination of fluorescence, MRI, and CT.
Drug Delivery Vehicle. NPs offer the potential to maximize the local efficacy of drugs while concurrently minimizing systemic
831 |
dx.doi.org/10.1021/bc200065z |Bioconjugate Chem. 2011, 22, 825–858 |

Bioconjugate Chemistry |
|
REVIEW |
Figure 5. An example of drug delivery using polymeric NPs. (a) Poly(D,L-lactic-co-glycolic acid) or PLGA NPs were modified with prostate specific membrane antigen binding aptamer (Apt) and encapsulated the drug Docetaxel (Dtxl). (b) Quantitative and (c) qualitative changes in tumor size following administration of di erent formulations. The Dtxl-NP-Apt conjugate was the most e cacious, followed by the untargeted Dtxl-NP. The Dtxl alone was the least e cacious. Figure adapted with permission from ref 129. Copyright 2006 National Academy of Sciences, USA.
toxicity, thereby improving the quality of life and prognosis for patients undergoing chemotherapy. For example, Farokhzad et al. demonstrated that polymer NP-bioconjugates could increase the efficacy of chemotherapeutic treatment in mouse tumor models.129 The drug Docetaxel was encapsulated within NPs composed of PEG-PLGA and conjugated with nucleic acid aptamers that targeted prostate specific membrane antigen. As shown in Figure 5, when compared to treatment with only Docetaxel, the NP(Docetaxel)-aptamer conjugates were found to cause concomitant and substantial reductions in both tumor size and treatment toxicity. Although treatment with NP(Docetaxel) was more efficacious than only Docetaxel, it was less than that with NP(Docetaxel)-aptamer conjugates, highlighting the importance of adding targeting to the NP-drug vector via the aptamer.
Theranostic Agent. “Theranostics” describes the combination of diagnostic and therapeutic utility in a single entity and has the potential to significantly enhance the treatment of disease. NPs have two important features that are highly favorable to theranostic application: (1) dimensionality that can allow cellular delivery and biodistribution and (2) nontrivial surface area or volume that can be derivatized with potentially many different molecules to impart a variety of functions (e.g., targeting, delivery). As shown in Figure 6, Cheng et al. recently reported the synthesis of trifunctional mesoporous silica NPs for theranostics.130 The NP surface was decorated with cyclic Arg- Gly-Asp (cRGDyK) peptides to target the NPs to overexpressed integrins on cancer cells, and near-infrared fluorescent dye molecules were incorporated within the silica framework for visualization. In addition, a photosensitizer used in photodynamic therapy was loaded into the nanopores of the silica NP. Cellular studies indicated a high degree of targeting specificity and cytotoxicity. In some cases, NPs can also offer intrinsic features for theranostic purposes. For example, QDs allow the direct sensitization of
conjugated photodynamic therapy drugs attached to them, while Au NPs can provide concomitant photothermal effects.131,132
The examples above highlight representative uses of several NP materials in di erent applications. For each material, many more applications have been reported, and similarly, for each application, many other NP materials have been used. Nonetheless, in nearly all cases, the controlled preparation of NP-conjugates is necessary to e ectively combine and use the unique properties of
both the NP material and the biomolecule for the intended application.
’NANOPARTICLES AND BIOCONJUGATE CHEMISTRY
Challenge of Nanoparticles. It is difficult to generalize the many challenges associated with preparing NP bioconjugates. The diversity of NPs is such that dimensionality can vary by 2 orders of magnitude (1 100 nm) between materials, with each exhibiting different degrees of polydispersity. Nonetheless, several common features are important to recognize:
•NPs are large compared to most molecules.
•NPs are su ciently small to be functionalized for colloidal solubility and are able to di use.
•NPs have nontrivial surface area and can occupy or contain nontrivial volumes that vary over a wide range of magnitudes based on NP size.
•NPs tend to exhibit heterogeneity across a population.
NPs thus have a character that is intermediate between molecules and bulk interfaces. Arguably, the closest molecular analogue for a NP is a protein. Proteins are large compared to most other molecules and are soluble, adopting a threedimensional structure where the character of the amino acid residues exposed to solution largely determines protein solubility and the potential labeling methods. It is thus not surprising that much of the initial work in the preparation of NP-bioconjugates drew inspiration from standard protein labeling chemistries, such as those shown Figure 7. Despite the conceptual similarity between NPs and proteins, there are important di erences between protein labeling and NP bioconjugation. Consider the labeling of proteins with fluorescent dyes: excesses of reactive dyes are frequently used to shotgun label proteins, and these approaches are often e ective given the monoreactivity of the dyes and the availability of many reactive amino acid residues (e.g., lysine) at the surface of a folded protein. The degree of labeling is measured experimentally and the reaction optimized empirically. Since the size of the dye label is comparable to the size of the amino acid residues, the perturbation of the protein tends to be minimal. However, as outlined above, NPs are quite dissimilar to dye molecules by virtue of the nontrivial size and functionalization with polyreactive coatings.
832 |
dx.doi.org/10.1021/bc200065z |Bioconjugate Chem. 2011, 22, 825–858 |

Bioconjugate Chemistry |
|
REVIEW |
Figure 6. Trifunctional mesoporous silica NPs (MSNs) for theranostics. (a) Synthesis scheme showing incorporation of the Atto647N near-infrared fluorescent dye for tracking, surface modification with (3-aminopropyl)trimethoxysilane (APTMS), encapsulation of a Pd-porphyrin (PdTPP) photosensitizer for photodynamic therapy (PDT), and further surface modification with PEG and cyclic Arg-Gly-Asp (cRGD) for targeting Rvβ3 integrin.
(b) Theranostic scheme illustrating targeting, uptake, tracking, and localized PDT. (c) Two-color confocal fluorescence image of U87MG cells showing nuclei (blue: Hoechst dye) and MSN uptake (red: Atto647N). (d) U87MG toxicity data for targeted MSNs without (blue) and with (red) photoirradation, and for nontargeted MSNs without (green) and with (purple) photoirradiation. Figure adapted with permission from ref 130. Copyright 2010 Royal Society of Chemistry.
A good example of the potential liabilities of shotgun labeling methods is the popular cross-linking reaction between amines and carboxylic acids using carbodiimide activation (e.g., 1-ethyl- 3-(3-dimethylaminopropyl)carbodiimide, or EDC), shown in Figure 7c. It is often the case that a NP displays a high density of carboxylic acid groups and a target biomolecule of interest has one or more primary amines. One of the disadvantages of carbodiimide chemistry is that the reactive o-acylisourea intermediate formed through the activation of carboxylic acids is prone to rapid hydrolysis.1 As a consequence, a large excess of carbodiimide must be used to drive the desired cross-linking reaction to completion. Unfortunately, overactivation of the NP carboxyl groups—which often mediate aqueous solubility—can cause the loss of colloidal stability and aggregation due to the poor solubility of the o-acylisourea intermediate. This can be only partially ameliorated by converting the o-acylisourea to a more stable reactive intermediate using N-hydroxysuccinimide (NHS) or a sulfonated derivative thereof.1 A sulfonated succinimidyl ester intermediate has greater solubility and helps maintain
colloidal stability during the reaction. Although the succinimidyl ester intermediate is not resistant toward hydrolysis, it hydrolyzes more slowly than the o-acylisourea intermediate, and this tends to increase reaction e ciency. The conversion of the o-acylisourea intermediate to a (sulfo)fluorophenyl ester provides yet greater resistance toward hydrolysis133 but, like NHS, is still limited by the dependence on EDC for in situ activation. Although EDC-driven coupling is a very powerful and popular chemistry that is often made to work with NPs, it is critical to realize that successful use of this shotgun cross-linking between a carboxylated NP and an aminated biomolecule (or vice versa) is
not equivalent to controlling the display of that biomolecule on the NP.134
There are several criteria that define ideal chemistries for the controlled display of biomolecules on NPs, and these can often be di cult to realize in practice:
•The average number or ratio of biomolecules per NP (i.e., the conjugate valence) is both predictable and reproducible on the basis of reaction stoichiometry.
833 |
dx.doi.org/10.1021/bc200065z |Bioconjugate Chem. 2011, 22, 825–858 |
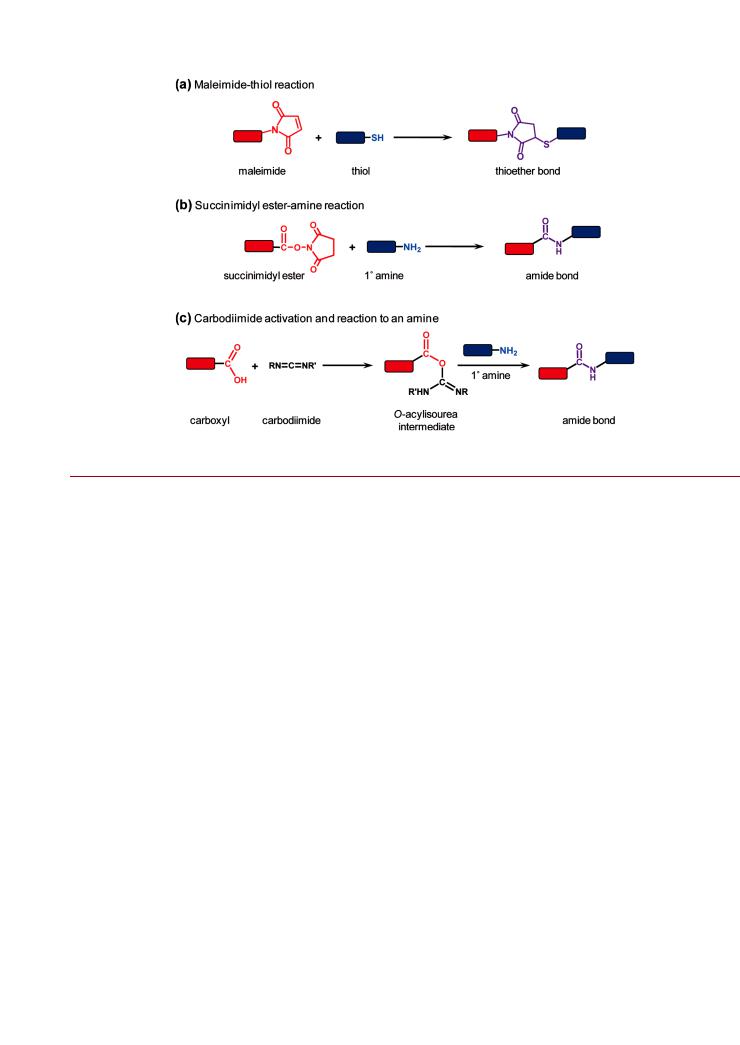
Bioconjugate Chemistry |
|
REVIEW |
Figure 7. Standard bioconjugation reactions, including (a) maleimide thiol, (b) succinimidyl ester-amine, and (c) carbodiimide-mediated coupling between carboxyls and amines.
•The NP-conjugates have minimal polydispersity in their valence across the ensemble.
•The attachment point of a biomolecule to the surface of the NP is both predictable and reproducible.
•The distance between the NP to a given moiety of a biomolecule is both predictable and reproducible.
•A corollary of the previous two points is that biomolecular orientation is controlled.
•The activity of a biomolecule or the e cacy of a small molecule is minimally compromised by its attachment to a NP. Similarly, NP functionality is not compromised.
•The linkage between the NP and the biomolecule is stable under most conditions, but is potentially labile under selected conditions (i.e., drug delivery). Stability or lability as a function of time, temperature, and pH may be important.
•The bioconjugate reaction proceeds to completion rapidly (<1 h) under mild aqueous conditions (i.e., does not require extremes of temperature or pH).
•The bioconjugate reaction is not prone to competing reactions (e.g., hydrolysis).
•The bioconjugate reaction is orthogonal to other bioconjugate chemistries (i.e., highly chemoselective).
•A corollary of the previous point is that the display of one type of biomolecule is compatible with the concurrent display of other biomolecules, as desired, using previous, parallel, or subsequent coupling reactions.
Given these considerations, it is clear that there are several challenges associated with the controlled display of biomolecules on NPs. Continuing with the example of carbodiimide coupling, polydispersity in NP size and surface area results in variability in the number of carboxylic acid groups available for activation per NP. This is exacerbated by the competition between overactivation and hydrolysis, which are very sensitive to reaction conditions
such as pH and temperature. Therefore, reaction stoichiometry does not directly translate into conjugate valence. In the case of certain synthetic NPs, it is possible to address this challenge through the chemistry used to coat the NP. The display of multiple functional groups or a “mixed” surface, where one group mediates solubility and other groups mediate reactivity, o ers a distinct advantage. Mei et al. prepared CdSe/ZnS quantum dots and Au NPs coated largely with unreactive methoxy-terminated PEG ligands, while also incorporating a small percentage of reactive carboxyl terminated PEG ligands.135 The PEG provides aqueous solubility while allowing the carboxyl groups to be fully converted into reactive intermediates via EDC. Thus, some control can be exercised over the final conjugates by driving the cross-linking reaction to saturation. However, control over the conjugate valence and its polydispersity must be achieved in the preparation of the coated NPs—the conjugate reaction itself is still not well controlled. Moreover, the strategy is best suited for the preparation of small molecule-NP conjugates: it is generally impractical to use large excesses of biomolecules in conjugate reactions with NPs. In addition to biomolecules being a more limited resource, biomolecule solubility can be limited (often to micromolar concentrations) and it may not always be possible to e ciently purify NP-bioconjugates from excess biomolecules (e.g., low conjugate valence and similar size between NP and biomolecule).
Another challenge that can be associated with using carbodiimide coupling and NPs is control over biomolecular orientation in the conjugate. Proteins often have many lysine residues that can participate in carbodiimide coupling. The size of the NP is also nontrivial, and the point of attachment is important for bioconjugate function. If the binding site of an antibody or enzyme is oriented toward and in close proximity to the surface of the NP, then some loss of activity can be expected. Indeed, this e ect of orientation has been observed with QD-antibody
834 |
dx.doi.org/10.1021/bc200065z |Bioconjugate Chem. 2011, 22, 825–858 |
