
Thermal Analysis of Polymeric Materials
.pdf
6 1 Atoms, Small, and Large Molecules
__________________________________________________________________
1.1.3 Classification Scheme for Molecules
In early classifications of types of molecules, one differentiated between organic and inorganic molecules. The former was thought to need the intervention of a living organism for their formation, the latter, not. Already in 1828 it was, however, discovered (accidentally) by Wöhler1 that urea, a typical organic molecule (H2N CO NH2), could be made in the laboratory from ammonium cyanate, an inorganic molecule (ONC NH4). With the blurred division between organic and inorganic molecules, it became the custom to distinguish biological molecules as truly organic molecules. Today one can even synthesize or modify biological molecules, removing much of the original usefulness for such classification.
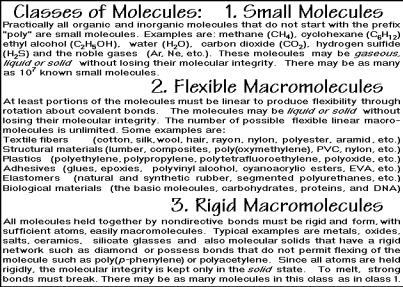
A better classification makes use of the sizes of molecules, dividing them into small molecules and macromolecules, as is indicated in Fig. 1.6 [3]. The importance of large molecules, or macromolecules, was shown by Staudinger2 about 80 years ago. Following his suggestion, any molecule with more than 1000 atoms or a molar mass of more than 10,000 Da should be called a macromolecule. This operational definition is quite useful since there are not many molecules that are known with about 1000 atoms. Most are either much larger or much smaller.
To complete this classification of Fig. 1.6, a further subdivision into flexible and rigid macromolecules is advantageous. Flexible macromolecules are of main interest
Fig. 1.6
1 Friedrich Wöhler (1800–1882) Professor at the Universities of Heidelberg and Göttingen in Germany.
2 Hermann Staudinger (1881–1965) Professor at the University of Freiburg, Germany, Nobel Prize for Chemistry in 1953.
1.1 Microscopic Description of Matter and History of Polymer Science |
7 |
__________________________________________________________________
to the discussions in this book and are usually, but not so precisely, just called polymers. The term plastics is often applied colloquially to the low-melting, polymeric materials. The polymers also are the macromolecules Staudinger introduced to chemistry as the last class of new molecules. The flexibility of such molecules is caused by internal rotation about at least some sigma bonds of proper geometry (see Sect. 1.3). Missing flexibility makes a macromolecule rigid. Rigid macromolecules are usually of the size of the phase, and make the distinction between phases and molecules superfluous and unwieldy. The molecule of a single crystal of 100 g, for example, reaches a molar mass of about 6×1019 metric tons.
Chemistry is the science dealing with synthesis, reactions, and properties of all substances, but traditionally small molecules have become the main focus. After the discovery of X-ray diffraction, rigid macromolecules have become the primary substances of interest to material science and solid state physics. The new, flexible macromolecules fell between these fields and have only slowly been accepted into the various academic disciplines. Presently polymer science is most prominently taught in material science courses, less in chemistry, and least in physics.
The key distinction between the three classes of molecules just described is summarized in the center of Fig. 1.6. Small molecules may appear in the solid, liquid, and gaseous phases without decomposition, while rigid macromolecules keep their bonding (molecular integrity) only to nearest neighbors in the solid state. Due to internal rotation, the flexible macromolecules can attain sufficient intramolecular disorder to melt (or dissolve) without breaking strong bonds. This property is at the root of many of the useful properties of polymers, as will be discussed throughout the book. The three classes of molecules are thus very distinct in their phase behavior.
No large molecules can be evaporated thermally without decomposition. If one tries to place flexible macromolecules into the gas phase by evaporation of the solvent molecules from a dispersion of droplets of a solution with only one macromolecule per droplet, the macromolecules become solid microphase particles and collect at the bottom of the container. Typical examples of single polymer glass phases and crystals are shown in Chap. 5.
A preliminary, operational definition of the solid state is given within the box of Fig. 1.6. It will be expanded upon and linked to the material properties throughout the book. For materials, the transitions between solid and liquid are basic and determine their utility. Similarly, the evaporation characteristics need to be known to choose a molecule for a given application. The new classification scheme for molecules of Fig. 1.6 is, thus, much more useful than the earlier, arbitrary distinction that relied upon the ability or inability of living organisms to synthesize a particular substance. The bottom brackets give a rather unique explanation of the glass transition which is detailed in Sect. 2.5.
Figure 1.7 contains a listing of small molecules, flexible macromolecules, and rigid macromolecules. The small molecules are the compounds of traditional chemistry, they are limited at the size of about 1000 atoms, as indicated in Fig. 1.6. As also pointed out in Fig. 1.6, they are often stable in all three phases, gas, liquid, and solid. One can see the importance of the different phases from terminology in use for hundreds of years. The compound H2O has three names, steam, water, and ice, which link it to its phases, a practice not carried to any other compound. Perhaps the
8 1 Atoms, Small, and Large Molecules
__________________________________________________________________
Fig. 1.7
difference between molecules and phases was understood by the time other important molecules were identified in detail.
Next, a series of flexible macromolecules is listed in Fig. 1.7 together with their applications. At sufficiently high temperatures they form mobile, random coils in a liquid state, as will be described in Sect. 1.3. On cooling, they become solid, either by crystallization (although they usually crystallize only partially) or by formation of a glass. These transitions are discussed in much more detail in Chaps. 2 and 5. If the flexible macromolecules are cross-linked, they show rubber elasticity, another unique polymer property, detailed in Sect. 5.6.5. Both, small molecules and flexible macromolecules must contain directive strong bonds, as shown in the Grimm tetrahedron of Fig. 1.5. These directive bonds connect the atoms to a molecule to a closed structure. Non-directive bonds with enough atoms, in contrast, lead to threedimensional, unlimited, open aggregates and, thus, rigid macromolecules.
Rigid macromolecules, the last group in Fig. 1.7, are often not recognized as separate molecules because of their enormous size. The molecule determines the size of the phase or vice versa. A single crystal of NaCl is naturally a single molecule. It is thus more important to know the structure, shape, and defects of the crystal than the mass of the macromolecule. The chemical formula refers only to the smallest unit describing the material and refers to a formula mass, not the molar mass. Similarly, the flexible macromolecules are represented by the formula of their repeating unit, not the full molecule, as will be discussed in Sect. 1.2. On fusion or sublimation, rigid macromolecules lose their integrity, as discussed above, and break into smaller units. A sodium chloride melt is thus not a liquid of small NaCl molecules, but rather a dynamic aggregate of positive sodium and chloride ions that constantly changes the bonding to their neighbors. Only in the gas phase are strongly polar, small NaCl molecules detectable.

1.1 Microscopic Description of Matter and History of Polymer Science |
9 |
__________________________________________________________________
1.1.4 The History of Covalent Structures
The historic development of knowledge about polymer science is rather surprising in that it took so long to recognize the existence of flexible macromolecules. This delay occurred despite the great importance of polymers to man. Flexible linear macromolecules are not only a key compound in material science, they are also the basic molecules of life in the form of proteins, nucleic acids, celluloses, and starches. Polymeric materials can be stronger than metals, be more (rubber-) elastic than any other substance, be among the best insulators of heat and electricity, but if properly chosen, can also conduct electricity. Polymeric adhesives, films, fibers, and packaging have thoroughly affected our way of life. Polymers provide the many enzymes, which govern the chemical reactions basic to life, supply the molecules that store and govern genetic information, and create the supporting structural basis of biological systems.
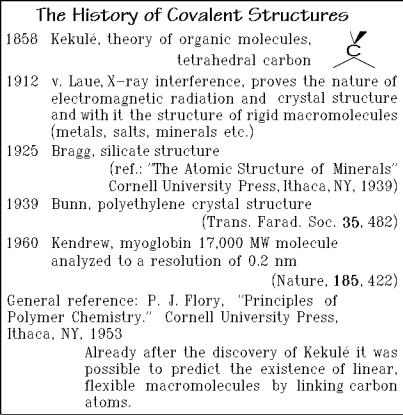
All flexible macromolecules must contain at least a sequence of linear, covalently bonded atoms. The understanding of the cylindrically symmetric, directed, covalent bonds (sigma bonds) was thus the first step towards polymer science. This step was already taken in 1858 by Kekulé1 as shown in Fig. 1.8. The detailed structures of large aggregates of atoms (macromolecules) could next be analyzed in detail after X-ray diffraction was fully understood. Major steps in the development of X-ray analysis of macromolecules are listed in the figure. First, rigid macromolecules such as metals, salts, minerals, and ceramics were analyzed. This was followed by many polymer crystal structures and even globular proteins.
The general reference to the book by Flory2 contains a brief, expert summary of the history of knowledge about polymers and is a treatise on polymer physical chemistry. (See also the list of general references for this section). The delay in development of polymer science due to the misunderstanding of colloid science (microphases) is discussed next.
1.1.5 The History of Natural Polymers
Naturally occurring polymers, as listed in Fig. 1.9, have always been present in nature and should have led to the early discovery of flexible, linear macromolecules. In retrospect, the observation of Gough, shown in Fig. 1.9, is already a hint at the thermodynamics of rubber elasticity, as will be shown in Chap. 5. The fact, that on extension polymers increase in temperature is an indication of a decrease in entropy. This contrasts the energy-elasticity of metals, which leads to constant or slightly decreasing temperatures on extension, indicative of unchanged or slightly increasing entropy on reversible extension (see Sect. 5.6).
Further development was, however, slowed with the discovery of colloids, as indicated in Fig. 1.9. The radii of colloidal particles are 10 4 10 6 cm. Today one
1 August Kekulé (1829–1896) Professor at the University of Bonn, Germany.
2 Paul John Flory (1910–1985) Professor at Stanford University, Nobel Prize for Chemistry, 1974 for work in the field of synthetic and natural macromolecules.
10 1 Atoms, Small, and Large Molecules
__________________________________________________________________
Fig. 1.8
would call phases of such dimensions microphases (with less than one micrometer in size, 10 4 cm) or nanophases (when approaching one nanometer in size 10 7 cm). Flexible macromolecules are by themselves already in the range of colloidal dimensions, i.e., they may be longer than the diameter of a microphase. Many of the properties of polymers are due to the possibility that a single molecule may, thus, be part of more than one phase and cause strong interactions across the phase boundaries.
On the example of natural rubber listed in Fig. 1.9 it can be seen how the knowledge about colloids has hindered the development of polymer science. The series of the listed experiments should have made it increasingly clear that rubber consists of long-chain molecules. Instead, it was concluded at the turn of the 19th to the 20th century that there exists no single molecule with the polymer structure, but that rubber is a colloidal phase of weakly attached rings. Covalently bonded molecules of colloidal dimensions were thought to be impossible. The assumption that the basic rubber molecules were rings was needed to account for the simple C5H8 stoichiometry. Macromolecules are a typical example of the difficulties one has to change prevalent opinion, even in the natural sciences.

1.1 Microscopic Description of Matter and History of Polymer Science |
11 |
__________________________________________________________________
Fig. 1.9
1.1.6 The History of Synthetic Polymers
The many synthetic polymers or flexible macromolecules are practically all new molecules on earth, and as with any new substance, there may be a hazard to the environment on its introduction in large volume. Fortunately, as soon as one becomes aware of problems with a specific polymer, a remedy can be found by altering the polymer produced because of the multitude of available macromolecules, as described in Chap. 3. The enormous usefulness of the materials by far outweighs any of the temporary problems that have been created and will arise in the future. The use of polymers has changed technology on a similar scale as the availability of cheap iron some 200 years ago, which was the major root of the industrial revolution (1750–1900).
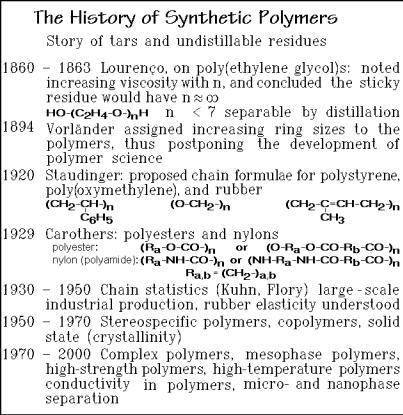
The history of synthetic polymers started with the analysis of sticky and tar-like residues left after organic syntheses. A typical example was the early work on poly(ethylene glycol) shown in Fig. 1.10. The logical progress to polymer science would have been faster if microphases and molecules would have been better distinguished, as is suggested in the figure. Only in 1920, through the large effort of
12 1 Atoms, Small, and Large Molecules
__________________________________________________________________
Fig. 1.10
Staudinger [4], was it finally established without doubt, that flexible, linear macromolecules are a new class of molecules as was described in Sect. 1.1.3. With the discovery of this new class of molecules the overall description of all molecules is possible as summarized in Figs. 1.6 and 7.
Once discovered and understood, the number of new polymers and knowledge about them grew rapidly. Some of the milestones are listed also in Fig. 1.10. The growth of new information on polymers went parallel with the enormous growth of the polymer industry. It is surprising that the traditional academic disciplines were very slow in including the new body of knowledge about molecules into their field of interest. In fact, many of the premier universities in the world still do not have polymer chemistry or physics in their curriculum. Much of the early progress of polymer physical chemistry and physics was thus left to the organic chemists who made the new molecules and the material and textile engineers who had to support the new industrial uses. The basics of nomenclature and structure, as well as descriptions of the molecular size-distribution and the experiments needed to experimentally characterize the molecular sizes of the flexible macromolecules are discussed in the remaining sections of this chapter.
1.2 Nomenclature |
13 |
__________________________________________________________________
1.2 Nomenclature
1.2.1 Sourceand Structure-based Names
Flexible macromolecules were identified in Fig. 1.6 within the framework of all possible molecules, and the historical development of the science of the field was sketched. The present topic is nomenclature. Without mastery of the basic nomenclature it is difficult to talk about any new subject matter, much less is it possible to learn its intricacies. For this reason, one must go through the drudgery of learning the needed names and rules by rote. Without such drill, future discussions will have to be at a much lower level. When discussing macromolecules, their name and their structure must be in ones mind in order to understand their behavior. Only then, for example, can there be a link between the question of flexibility, reaction mechanisms, and crystallization to the bonds and atoms that are affected.
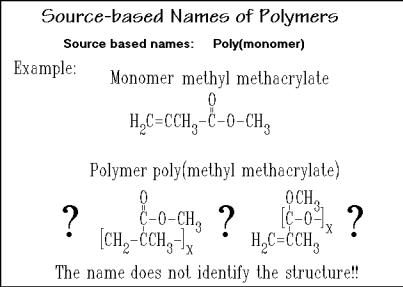
The rules of nomenclature are set by the International Union of Pure and Applied Chemistry (IUPAC) [5]. When a new polymer has been made, a need to name it arises, even before the structure is known. This is done by giving the polymer a source-based name, as indicated in Fig. 1.11. For example, the polymer made by polymerization of methyl methacrylate is called poly(methyl methacrylate). The monomer name is simply added to the word poly (Gk. = many; note that
Fig. 1.11
monomer stems from = single, and = part). If the name of the monomer consists of only one word, the parentheses can be omitted, as for example in polyethylene or polypropylene. Often this simple, source-based name, once given, remains in common usage. One notices immediately that some basic knowledge of

14 1 Atoms, Small, and Large Molecules
__________________________________________________________________
nomenclature of small molecules is required to deduce the polymer composition, and then to speculate about the possible structure.
In the case of methyl methacrylate and most other monomers, several different polymers could be made by different reactions, as will be discussed in Chap. 3. A structure-based name, thus, would be helpful in distinguishing polymers and understanding the special properties of the different macromolecules even if they arise from the same monomer.
The structure-based names are designed to give a precise link of the name to the structure. The rules are summarized in Fig. 1.12. Basic is the identification of the constitutional repeating unit (CRU). If there is no repetition of the structure within
Fig. 1.12
a macromolecule, a structure-based name may not be feasible, as has been experienced in efforts to name the enormous variety of proteins of which some examples are given in Chap. 3. First, the CRU must be identified by finding the repetition scheme of the molecular architecture along the linear molecule. Naturally, one could start with any atom in the backbone of the molecule and identify the CRU by the beginning of the next repetition cycle. To eliminate accidental multiplicity of names derived from beginning the naming from different atoms, a seniority list has been set up to fix the proper starting atom as is listed at the bottom of Fig. 1.12. Next, the CRU must be oriented properly. The name of an O C sequence in a CRU is different from C O. The appropriately chosen and oriented CRU can then be named. The names are based on the established rules of organic chemistry which can be looked up in any textbook. One needs, thus, to know a list of common diradicals that give the CRU when linked together. Finally, the end groups may have to be specified to complete the name of the molecule in the case of shorter molecules, as illustrated in Fig. 1.14, below.
1.2 Nomenclature |
15 |
__________________________________________________________________
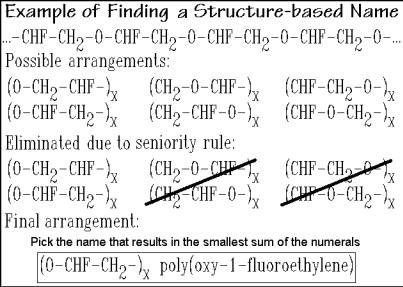
To illustrate these rules, a seemingly simple molecule is shown in Fig. 1.13 and the naming process is followed step by step. The structural formula being developed, the (CRU)x must indicate the continuation of the backbone bonds. This can be done by extending the bond through the parentheses, (CRU) x, an awkward way for typing. An easier way is chosen by placing the bond on the left fully outside of the parentheses and on the right fully inside the parentheses, (CRU )x. The left bond can then be omitted since it is not part of the repetition scheme of the CRU. Figure 1.13 shows that there are a total of six possible arrangements of CRUs starting with different atoms and going in the two different directions. The seniority list of Fig. 1.12 eliminates all but two.
Fig. 1.13
One additional rule, that of the smallest sum of numerals that are found in the name is to be added to find the name of the polymer: poly(oxy-1-fluoroethylene), as shown in the box of Fig. 1.13. Its monomer could be either a substituted ethylene epoxide or an ethylene glycol. The name of the not chosen CRU is poly(oxy-2- fluoroethylene) with the larger numeral in its name.
Figure 1.14 gives a short list of diradicals which are frequently found in polymers. Note that the name of the polymer should also be short. If there exists an acceptable name for a longer diradical, this name should be used. The diradical propylene should thus not be named (methylmethylene)methylene. Longer sequences of CH2- groups are called trimethylene, tetramethylene, etc. Naturally, ethylene, trimethylene etc. are not permitted as the only diradical in a CRU, such polymers are all poly(methylene)s. The structure-based names are always enclosed in parentheses, even if the diradical is a single word, as in poly(butenylene).
Next, a much more complicated example is illustrated in Fig. 1.14. Poly(vinyl butyral) is produced by making poly(vinyl alcohol) and then reacting it with
