
ААС
.pdf
This article was downloaded by: [Mount Royal University] On: 23 May 2013, At: 07:03
Publisher: Taylor & Francis
Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK
Analytical Letters
Publication details, including instructions for authors and subscription information:
http://www.tandfonline.com/loi/lanl20
Preparation of a Chelating Resin by Immobilizing 1- (2-Pyridylazo) 2-Naphtol on Amberlite XAD-16 and Its Application of Solid Phase Extraction of Ni(II), Cd(II), Co(II), Cu(II), Pb(II), and Cr(III) in Natural Water Samples
Ibrahim Narin a , Mustafa Soylak b , Kadriye Kayakirilmaz a , Latif Elci c & Mehmet Dogan d
aNigde University, Faculty of Art and Science, Department of Chemistry, Nigde, Turkey
bErciyes University, Faculty of Art and Science, Department of Chemistry, Kayseri, Turkey
cPamukkale University, Faculty of Art and Science, Department of Chemistry, Denizli, Turkey
dHacettepe University, Faculty of Science, Department of Chemistry, Ankara, Turkey Published online: 02 Feb 2007.
To cite this article: Ibrahim Narin , Mustafa Soylak , Kadriye Kayakirilmaz , Latif Elci & Mehmet Dogan (2003): Preparation of a Chelating Resin by Immobilizing 1-(2-Pyridylazo) 2-Naphtol on Amberlite XAD-16 and Its Application of Solid Phase Extraction of Ni(II), Cd(II), Co(II), Cu(II), Pb(II), and Cr(III) in Natural Water Samples, Analytical Letters, 36:3, 641-658
To link to this article: http://dx.doi.org/10.1081/AL-120018254
PLEASE SCROLL DOWN FOR ARTICLE
Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae, and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand, or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.

Downloaded by [Mount Royal University] at 07:03 23 May 2013
ANALYTICAL LETTERS
Vol. 36, No. 3, pp. 641–658, 2003
SEPARATIONS
Preparation of a Chelating Resin by Immobilizing 1-(2-Pyridylazo) 2-Naphtol on Amberlite XAD-16 and Its Application of Solid Phase Extraction of Ni(II), Cd(II), Co(II), Cu(II), Pb(II), and Cr(III) in Natural Water Samples
Ibrahim Narin,1 Mustafa Soylak,2,* Kadriye Kayakirilmaz,1
Latif Elci,3 and Mehmet Dogan4
1Nigde University, Faculty of Art and Science, Department of Chemistry, Nigde, Turkey 2Erciyes University, Faculty of Art and Science, Department of Chemistry, Kayseri, Turkey 3Pamukkale University, Faculty of Art and Science, Department of Chemistry, Denizli, Turkey 4Hacettepe University, Faculty of Science, Department of Chemistry, Ankara, Turkey
*Correspondence: Mustafa Soylak, Erciyes University, Faculty of Art and Science, Department of Chemistry, 38039 Kayseri, Turkey; Fax: þ903524374933; E-mail: soylak@erciyes.edu.tr.
|
641 |
DOI: 10.1081/AL-120018254 |
0003-2719 (Print); 1532-236X (Online) |
Copyright & 2003 by Marcel Dekker, Inc. |
www.dekker.com |
Downloaded by [Mount Royal University] at 07:03 23 May 2013
642 |
Narin et al. |
ABSTRACT
A polystyrene divinylbenzene resin (Amberlite XAD-16) functionalized by 1-(2-pyridylazo) 2-naphtol has been synthesized and its sorption properties have been investigated for preconcentration of Ni(II), Cd(II), Co(II), Cu(II), Pb(II) and Cr(III) in natural water samples. The new resin (XAD-16-PAN) was characterized by thermogravimetric analysis and infrared spectrometry. The analytical parameters including pH, sample volume etc. for the quantitative recoveries of the analyte ions using XAD-16-PAN resin were investigated. The e ects of alkaline, earth alkaline ions and some anions on the sorption of analyte ions were also examined. The recovery values were greater than 95% and the preconcentration factor was 200 for all analyte ions. The relative standard deviations of the determinations by flame atomic absorption spectrometry were less than 8%. The detection limits of the analyte ions (k ¼ 3, N ¼ 21) were varying from 0.056 mg/L for Cd to 0.268 mg/L for Cr.
Key Words: Chelating Resin; Preconcentration; XAD-16-PAN;
Natural Waters; AAS.
INTRODUCTION
Separation and preconcentration techniques such as coprecipitation,
electrodeposition, solvent extraction, cloud point extraction, membrane filtration, solid phase extraction[1–5] for the trace heavy metal ions are
an important part of modern analytical chemistry. The most common technique available for the separation/preconcentration of metals from aqueous samples is solid phase extraction method using adsorbents such as sephiolite, thiol cotton, activated carbon, green tea leaves, microcrystalline naphthalene, cellulose, and Diaion HP-20.[6–11]
The syntheses of chelating resins from the commercial adsorption resins has been popular in the last decade.[12–18] In particular, studies
on the synthesis of resins by the reaction of a commercial resin and a suitable chelating agent and sorption characterization of chelating resin available for interesting metal ions have been extensively carried out in a number of analytical laboratories because both their sorption capacities and sorption selectivity are superior to those of ion exchangers in
trace levels. For that purpose, especially Amberlite XAD copolymers have been widely used.[19–24] Saxena and co-workers[19] have used
Amberlite XAD-2-Alizarin Red-S chelating resin for the preconcentration of lead(II), cadmium(II), zinc(II) and nickel(II). In another study,
Downloaded by [Mount Royal University] at 07:03 23 May 2013
Chelating Resin Preparation |
643 |
Pyrocatechol Violet immobilized Amberlite XAD-2 resin has been syn-
thesized by Saxena and Singh[20] and its adsorption characters has been identified. Dev et al.[15] established a new method for the sorption beha-
vior of lanthanum(III), neodymium(III), terbium(III), thorium(IV) and uranium(VI) on Amberlite XAD-4 resin functionalized with bicine ligands.
Amberlite XAD-16 is a polystyrene divinylbenzene copolymer. It has no ionizable functional groups. It has been used for the preconcentration
and separation of trace heavy metal ions from various media including natural waters, urine, dialysis concentrate, etc., by our group.[26–31] It is
used as a solid matrix for synthesis of chelating resin and separation of organic materials which has hydrophobic properties. The preparation of
new chelating resins by using Amberlite XAD-16 and some chelating agents have been performed by some researchers.[32–34] Lee and co-work- ers[32,33] synthesized a new chelating resin by using Amberlite XAD-16
and 4-(2-thiazolylazo) resorcinol. They investigated the adsorption properties of the new resin. Lee and co-workers[33,34] synthesized two di erent
chelating resins by using 4-(2-thiazolylazo) resorcinol and 1-(2-thiazoly- lazo)-2-naphthol as chelating agent and XAD-16 as support for the preconcentration of trace metal ions including uranium.
1-(2-pyridylazo) 2-naphtol (PAN) is a reagent for the spectrophotometric determination of lots of transition metal ions.[35,36] It acts as a
terdendate ligand complexing with metals through the hydroxyl oxygen
atom, pyridine nitrogen atom and one of the azo group nitrogen atoms.[35,36] This point is an important advantage of PAN. It has been
also used for the separation and preconcentration of trace heavy metals in various media. According to our knowledge, up to now, no systematic investigation on the preparation of chelating resin by combination of Amberlite XAD-16 and 1-(2-pyridylazo) 2-naphtol (PAN), the adsorption characteristics and its application in trace analysis have been performed.
In the present work, a chelating resin was synthesized by immobilizing 1-(2-pyridylazo) 2-naphtol (PAN) on Amberlite XAD-16 (XAD-16- PAN) and characterized. Its application of preconcentration of some metal ions in natural water samples was performed. The optimal analytical conditions including pH, resin amounts, flow rates of sample and elution solutions and matrix e ects were also investigated.
|
EXPERIMENTAL |
|
|
|
Apparatus |
|
|
Shimadzu |
6501F AAS flame |
atomic |
absorption spectrometer |
was employed |
for determination of |
metal |
ions. Atomic absorption |
Downloaded by [Mount Royal University] at 07:03 23 May 2013
644 Narin et al.
measurements were carried out in air/acetylene flame. The operating parameters for working elements were set of these recommended by the manufacturer. The nebulizer uptake rate was adjusted to give the optimum response for conventional sample introduction, the resulting rate being 6.0 mL/min. An acetylene flow rate of 2.5 L/min was used with an airflow rate of 8.0 L/min.
Thermo-gravimetric analysis was performed on a Shimadzu DT-40 model equipped with DTA and TG units. IR spectra were recorded on a Jasco 300 FTIR spectrometer. A mechanical shaker was used for batch experiments.
Reagents
All solutions were prepared with deionized water. Unless otherwise stated analytical-grade acetone, acids and other chemicals used in this study, were obtained from Merck, Darmstadt, Germany. Stock solutions of all metals, containing 1000 mg/L (Merck, Darmstadt) were used for preparation of the standards for the calibration curve. The calibration standards were not submitted to the preconcentration procedure.
The range of the calibration standards for Ni(II), Cd(II), Co(II), Cu(II), Pb(II), and Cr(III) on flame atomic absorption spectrometric determinations were 0.5–10.0, 0.02–2.0, 0.2–5.0, 0.5–10.0, 1.0–20.0, and 0.5–10.0 mg/L, respectively. The correlation coe cient of the calibration curves were generally 0.999.
Ammonium acetate bu er solutions (0.1 M) were prepared by adding an appropriate amount of acetic acid (Merck, Darmstadt) to ammonium acetate solutions for pH 2–6 and ammonium chloride bu er solutions (0.1 M) were prepared by adding an appropriate amount of ammonia (Merck, Darmstadt) to ammonium chloride solutions for pH 8–10.
1-(2-pyridylazo) 2-naphtol (PAN) was purchased from Sigma Chem. Co., St. Louis. Amberlite XAD-16 resin (Sigma Chem. Co.) was purchased as 20–40 mesh (specific area: 750 m2/g).
Synthesis of Chelating Resin
The procedure given by Saxena et al.[19] with some little modifications was applied to the synthesis of the XAD-16-PAN chelating resin. 10.0 g of Amberlite XAD-16 was treated with a nitrating mixture, containing 20 mL of concentrated nitric acid and 50 mL of concentrated sulfuric acid and stirred for 1 h at 60 C on a water-bath. The nitrated
Downloaded by [Mount Royal University] at 07:03 23 May 2013
Chelating Resin Preparation |
645 |
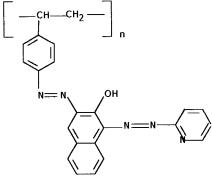
Figure 1. Formula of new chelating resin.
mixture was poured into ice-cold water. It was further filtered, washed repeatedly with distilled water until free from acid. Then it was reduced with SnCl2 (40 g), concentrated hydrochloric acid (45 mL), and ethanol (60 mL), and refluxed for 12 h at 40 C. The amino polymer was filtered o and washed with mixture of HCl-ethanol, water, and 2 M NaOH so as to get the free amino polymer.
The amino polymer was treated with 100 mL of 2 M HCl for 30 min, washed with distilled water in order to remove excess of HCl, suspended in 250 mL of ice cold water and mixed with 1 M HCl and 1 M NaNO2 in aliquots of 2.0 mL each time with constant stirring. The diazotized resin was filtered and washed with ice-cold water. It was reacted with 1-(2-pyridylazo) 2-naphtol (2.0 g taken in 100 mL solution in water/ethanol mixture) at room temperature for 1 h. The resulting resin was filtered and washed with distilled water. The formula of XAD-16- PAN resin is given in Fig. 1.
Column Preparation
The glass column, having a stopcock and a porous disk, was 10 cm long, and 1.0 cm in diameter. A small amount of glass wool was placed on the disc to prevent disturbing of the XAD-16-PAN resin beads during sample loading. Then, 600 mg of XAD-16-PAN chelating resin was slurried in water, and then poured into the column. The bed height of resin in the column was approximately 20 mm. It was washed successively with water, acetone, and water, respectively, then conditioned with 10–15 mL of bu er of pH 9. After each use, the resin in the
Downloaded by [Mount Royal University] at 07:03 23 May 2013
646 |
Narin et al. |
column was washed with large volumes of water and stored in water for the next experiment.
Preconcentration Procedure
The XAD-16-PAN column method was tested with model solutions prior to the determination of trace metal ions in seawater samples. For the metal determinations 100 mL of solution containing 10–20 mg of the metal ions was added to 10 mL of bu er solution (desired pH between 1 and 11). The column was preconditioned by passing bu er solution. The bu ered metal solution was passed over the column at a flow rate of 4 mL/min by the aid of a vacuum aspirator. After passing of this solution, the column was rinsed twice with 10 mL of water. The adsorbed metals on the column were eluted with 8–10 mL portion of 2 M HCl in acetone. The eluent was evaporated over a hot plate to near dryness. The residue is dissolved with 5 mL of 1.0 M HCl. The eluent was analyzed for the determinations of metal concentrations by flame AAS.
Analysis of Water Samples
The water samples were collected in prewashed (with detergent, doubly de-ionised distilled water, dilute HNO3 and doubly deionised distilled water respectively) polyethylene bottles. The samples were filtered through a Millipore cellulose nitrate membrane of pore size 0.45 mm. The samples were stored in 1 L polyethylene bottles and acid-
ified to 1% with nitric acid and were subsequently stored at 4 C in a refrigerator.[37,38]
For application of the method, 1000 mL of water sample was taken in a beaker, then the pH of the sample was adjusted to pH 9 with ammonia/ammonium bu er solution. The separation/preconcentration method given above was applied. The concentrations of the investigated analyte ions in the final solution were determined by flame AAS.
RESULTS AND DISCUSSION
Characteristics of XAD-16-PAN Chelating Resin
The thermogravimetric analysis (TGA) curve of the XAD-16-PAN chelating resin shows two steps. In the first step, a mass loss of 2.02%
Downloaded by [Mount Royal University] at 07:03 23 May 2013
Chelating Resin Preparation |
647 |
up to 121.2 C is due to adsorbed water on the resin. In the second step
mass loss is 6.75% up to 346.6 C. These situations agree with the previous studies.[19–21,24,25] When the infrared spectra Amberlite XAD-16
and XAD-16-PAN resins were compared, in the chelating resin two additional bands at 1629 and 3450 cm 1 that may be assigned to azo
(–N¼N–) and phenolic O–H stretching vibrations. These results also agree with the previous studies.[19–21,24,25]
Six mini columns containing 600 mg of XAD-16-PAN resin for each investigated ions were prepared and saturated with each metal ions, separately in the optimal conditions. The columns were washed with bu er solution and water, respectively, and then the saturated resins were dried on air. Their IR spectra were recorded and compared with IR spectra of XAD-16-PAN resin. The band (1629 cm 1) due to azo group undergoes a red shift of 8–15 cm 1 when a metal ion was adsorbed on the XAD-16-PAN resin. This suggests that the coordination of the metal ions with azo group is very much responsible for their sorption onto the XAD-16-PAN resin.
E ects of pH on the Retentions
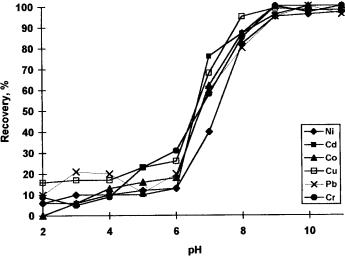
The influence of pH on the retentions of Ni(II), Cd(II), Co(II), Cu(II), Pb(II) and Cr(III) on the XAD-16-PAN resin were investigated in the pH range of 2–11. For this purpose column experiments were performed by using 50–60 mL of test solutions containing 10–20 mg of each analyte ions. The pHs of the model solutions were adjusted by using bu er solutions given in the experimental section. The results are depicted in Fig. 2. The investigated ions were quantitatively (>95%) recovered for all metal ions in the pH range of 9–11 except copper. The recoveries of copper were quantitative in the pH range of 8–11. The volume of the bu er solution had no e ect. In all further studies, 10 mL ammonium chloride bu er solution for pH 9 was used.
The model studies at pH 9 and 10 were also repeated by adjusting the pH of the solutions with 1 M NaOH. While at pH 9, cobalt and copper were quantitatively recovered with 1 M NaOH, quantitative values were obtained at pH 10 for cadmium, cobalt, copper and lead.
Influences of Eluent Type and Eluent Volume
The e ects of eluent type are an important factor in the solid phase extraction studies. The e ects of 10 mL of various eluents on the
Downloaded by [Mount Royal University] at 07:03 23 May 2013
648 |
Narin et al. |
Figure 2. E ects of pH on the retentions of analyte ions on XAD-16-PAN resin (N ¼ 4, eluent: 2 M HCl in acetone).
recoveries were studied. The results are given in Table 1. The quantitative recoveries of Ni, Cd, Co, Cu, Pb, and Cr were obtained for all analyte ions by using 2 M HCl in acetone as an eluent. However selective recoveries of metal ions were also possible. For example 1 M HCl was used as eluent for nickel, cobalt and lead quantitatively separated from other analyte ions. Also Ni, Co and Cu ions were quantitatively recovered by 1 M HNO3 as eluent.
The e ects of volume of 2 M HCl in acetone as an eluent were also investigated in the range of 5–10 mL. Quantitative recovery values (>95 %) for analytes were obtained after 6.0 mL of 2 M HCl in acetone. In all further studies, 7.0 mL of 2 M HCl in acetone was used as eluent.
E ects of Resin Amount
The influences of the amount of XAD-16-PAN resin on the mini column were also investigated in the range of 300–700 mg (Fig. 3). The recovery values for the investigated ions were not quantitative till 500 mg of the resin. With more than 500 mg of XAD-16-PAN resin quantitative recovery values obtained. In all subsequent studies 600 mg of XAD-16-PAN resin was used.

Downloaded by [Mount Royal University] at 07:03 23 May 2013
Chelating Resin Preparation |
649 |
Table 1. Influences of various eluents on the recoveries of analyte ions from the XAD-16-PAN column (N:4, eluent volume: 10 mL).
|
|
|
|
|
Recovery (%) |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||||
Eluent type |
|
Ni |
Cd |
Co |
Cu |
Pb |
Cr |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
1 M HNO3 |
101 |
2 |
87 2 |
93 |
3 |
94 |
2 |
78 |
4 |
88 |
3 |
|
2 M HNO3 |
69 |
3 |
72 2 |
68 |
3 |
84 |
2 |
73 |
3 |
73 |
1 |
|
1 M HNO3 in acetone |
87 |
2 |
92 3 |
102 |
2 |
99 |
3 |
95 |
2 |
103 |
1 |
|
2 M HNO3 in acetone |
90 |
2 |
80 3 |
86 |
2 |
84 |
3 |
82 |
3 |
77 |
3 |
|
1 M HCl |
100 |
2 |
77 2 |
98 |
2 |
60 |
3 |
101 |
2 |
88 |
4 |
|
2 M HCl |
89 |
3 |
67 2 |
94 |
2 |
92 |
3 |
103 |
2 |
77 |
3 |
|
1 M HCl in acetone |
103 |
2 |
89 2 |
100 |
3 |
104 |
2 |
101 |
3 |
85 |
2 |
|
2 M HCl in acetone |
101 |
2 |
95 2 |
101 |
3 |
98 |
2 |
103 |
2 |
95 |
2 |
|
1 M NH3 |
<10 |
<10 |
<10 |
17 |
2 |
<10 |
<10 |
|||||
2 M NH3 |
<10 |
<10 |
<10 |
16 |
2 |
<10 |
<10 |
|||||
1 M NH3 in acetone |
<10 |
<10 |
13 |
1 |
21 |
2 |
11 |
2 |
<10 |
|||
2 M NH3 in acetone |
<10 |
<10 |
<10 |
18 |
3 |
<10 |
<10 |
|||||
Figure 3. E ects of resin amounts on the recoveries of investigated ions (N ¼ 4, eluent: 2 M HCl in acetone).
